User login
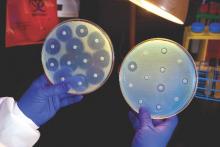
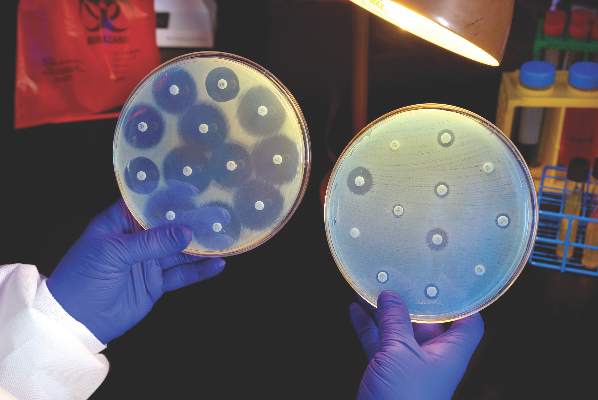
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Ceftazidime-avibactam effectively treated carbapenem-resistant Enterobacteriaceae (CRE) infections, but resistance emerged rapidly and in some cases led to microbiological failure.
Major finding: The rate of clinical success was 59%; 10% of patients developed acute kidney injury within 7 days of starting treatment; 8% developed resistance.
Data source: A single-center retrospective study of 37 patients with carbapenem-resistant Enterobacteriaceae infections treated with ceftazidime-avibactam.
Disclosures: The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. A coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.