User login
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
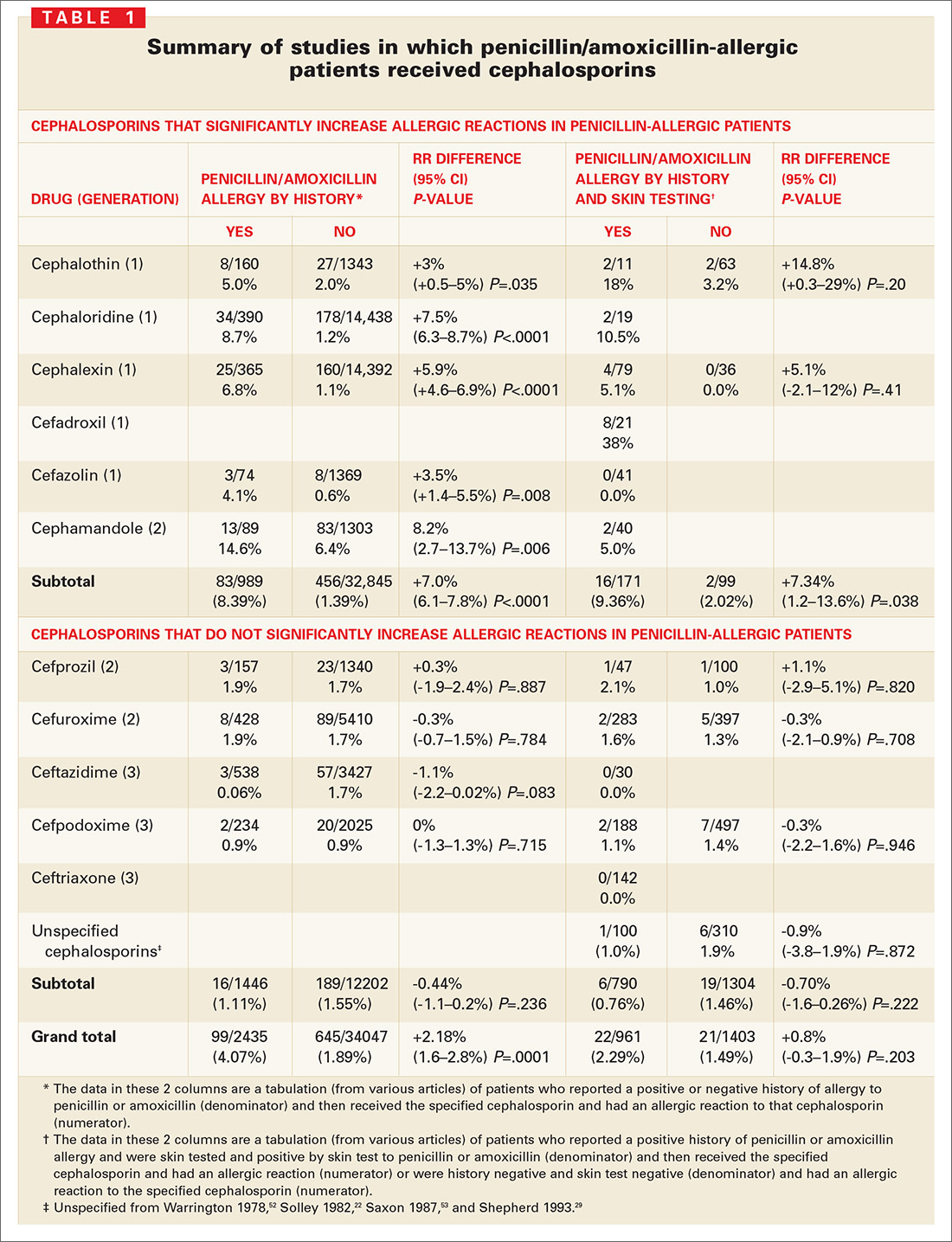
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).
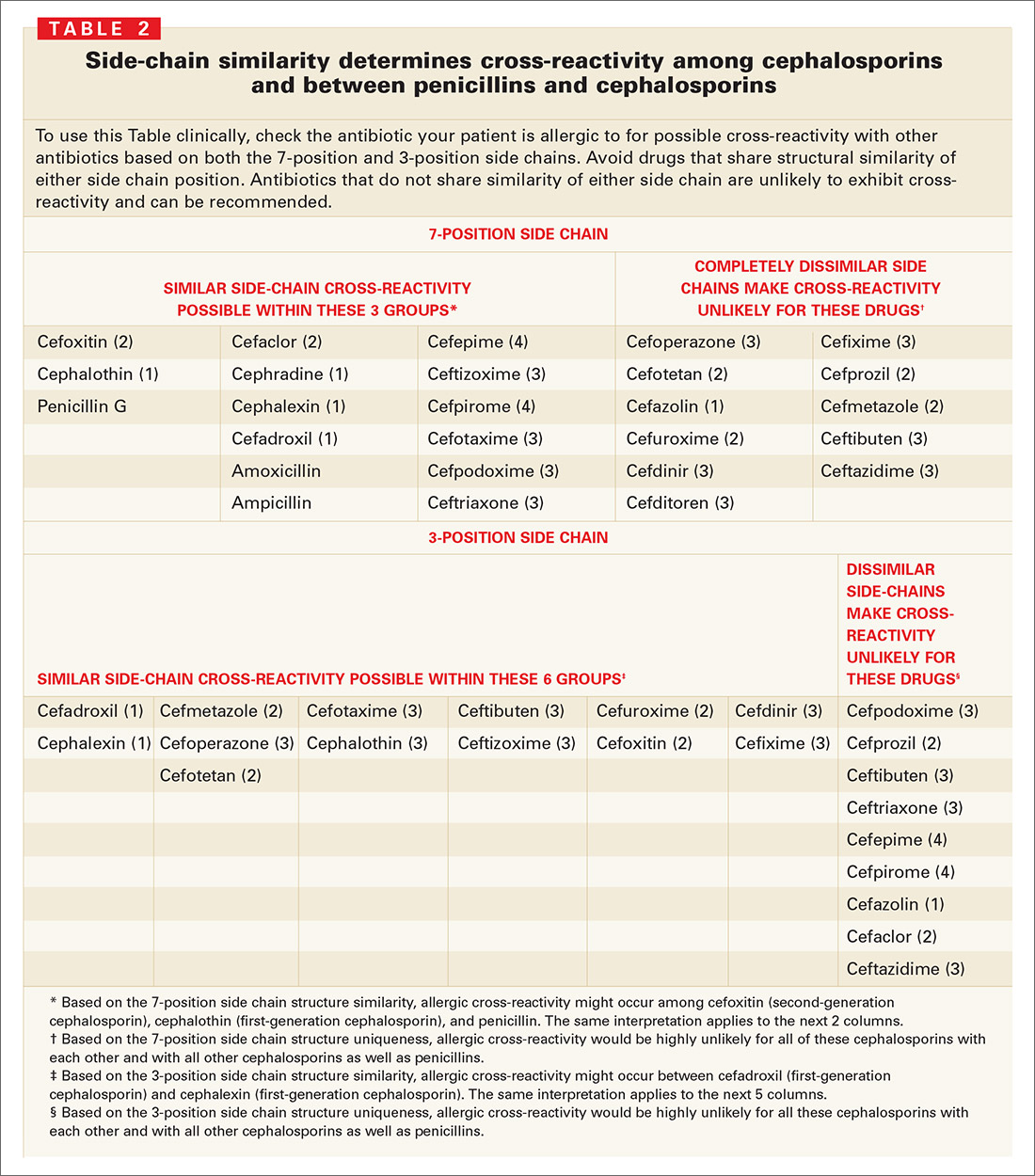
Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.
Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
- The widely quoted cross-allergy risk of 10% between penicillin and cephalosporins is a myth (A).
- Cephalothin, cephalexin, cefadroxil, and cefazolin confer an increased risk of allergic reaction among patients with penicillin allergy (B).
- Cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone do not increase risk of an allergic reaction (B).
Undoubtedly you have patients who say they are allergic to penicillin but have difficulty recalling details of the reactions they experienced. To be safe, we often label these patients as penicillin-allergic without further questioning and withhold not only penicillins but cephalosporins due to concerns about potential cross-reactivity and resultant IgE-mediated, type I reactions. But even for patients truly allergic to penicillin, is the concern over cephalosporins justified? It depends on the specific agent. What is certain is that a blanket dismissal of all cephalosporins is unfounded.
The truth about the myth
Despite myriad studies spanning decades and involving varied patient populations, results have not conclusively established that penicillin allergy increases the risk of an allergic reaction to cephalosporins, compared with the incidence of a primary (and unrelated) cephalosporin allergy. Most people produce IgG and IgM antibodies in response to exposure to penicillin1 that may cross-react with cephalosporin antigens.2 The presence of these antibodies does not predict allergic, IgE cross-sensitivity to a cephalosporin. Even penicillin skin testing is generally not predictive of cephalosporin allergy.3
Reliably predicting cross-reactivity
A comprehensive review of the evidence shows that the attributable risk of a cross-reactive allergic reaction varies and is strongest when the chemical side chain of the specific cephalosporin is similar to that of penicillin or amoxicillin.
Administration of cephalothin, cephalexin, cefadroxil, and cefazolin in penicillin-allergic patients is associated with a significant increase in the rate of allergic reactions; whereas administration of cefprozil, cefuroxime, cefpodoxime, ceftazidime, and ceftriaxone is not.
Penicillin skin testing can accurately predict a penicillin-allergic reaction, but is not predictive for cephalosporin allergy unless the side chain of the penicillin or ampicillin testing reagent is similar to the cephalosporin side chain being evaluated. Patients who have a reaction to a penicillin or a cephalosporin that is not IgE mediated and not serious may receive repeated courses of that antibiotic and related antibiotics.
This article provides a comprehensive review of the frequency of allergic cross-reactivity between penicillin/amoxicillin and cephalosporin antibiotics, supporting the recent American Academy of Family Physicians evidence-based clinical practice guideline on treatment of acute otitis media recommending the use of cefuroxime, cefpodoxime, cefdinir, and ceftriaxone cephalosporins for patients allergic to penicillin.
Methods
We searched Medline and EMBASE databases for English-language articles using the keywords cephalosporin, penicillin, allergy, and cross-sensitivity for the years 1960 to 2005. Among 219 articles identified, 101 were included as source material for this review. Articles we excluded were reviews, republication of results, or ones irrelevant to our purpose.
Five articles described the rate of rashes following use of penicillin and cephalosporins,4-8 and 4 articles described rates of anaphylaxis.5,9-11 We included 26 articles for the evidence base evaluating penicillin/amoxicillin cross-allergy.3,12-36 Eleven articles relied on patient history of penicillin/amoxicillin allergy to categorize results and establish reaction rates and relative risks for the penicillin/amoxicillin allergic vs nonallergic when receiving cephalosporins.12-15,17-20,27,28,31 Fourteen articles relied on patient history of penicillin/amoxicillin allergy plus skin testing results to penicillin/amoxicillin to categorize patients.16,21-25,29,30,32-37 One article3 provided data on a subset where penicillin/amoxicillin allergy was established based on history, and a separate subset where penicillin/amoxicillin allergy was established by skin testing. Other articles related to antibiotic chemical structures, animal studies, monoclonal antibody studies, cross-reactive antibody studies, and antibiotic skin testing were also reviewed.
Results
True incidence of reactions to cephalosporins
The most frequent reactions to cephalosporins are non-pruritic, non-urticarial rashes, which occur in 1.0% to 2.8% of patients;4-8 for most, the mechanism is idiopathic and not a contraindication for future use.38 Retrospective studies suggest a 1% to 3% incidence of immune or allergic reactions to cephalosporins independent of any history of penicillin/amoxicillin allergy.31 Anaphylactic reactions from cephalosporins are extremely rare, with the risk estimated at 0.0001% to 0.1%.31,38 A seminal study suggested approximately 0.004% to 0.015% of treatment courses with penicillin results in anaphylaxis.5,9-11 Several studies suggest that cephalosporin-induced anaphylaxis occurs no more frequently among patients with known penicillin allergy than among those without such allergy.23,27,38-41
Determining cross-reactivity
Penicillins and cephalosporins both possess a beta-lactam ring for antimicrobial activity. They differ in that the 5-membered thiazolidine ring of penicillin is replaced in the cephalosporins with a 6-membered dihydrothiazine ring. After degradation, penicillin forms a stable ring, whereas cephalosporins undergo rapid fragmentation of their rings.42 Immunologic cross-reactivity between the penicillin and cephalosporin beta-lactam rings is, therefore, very unlikely—an observation confirmed by monoclonal antibody analysis.43
How the “10% cross-reactivity” myth took hold. When the first-generation cephalosporins cephaloridine and cephalothin were introduced in the 1960s, allergic and anaphylactic reactions were reported in patients with previous allergic reactions to penicillins. Subsequent reports, which attributed up to 10% cross-reactivity between the 2 drug classes, involved these same first-generation cephalosporins plus cephalexin and cefadroxil and a second-generation drug, cefamandole. However, these studies were flawed because the penicillin test compounds had been contaminated with cephalosporins. Until 1982, penicillin was produced commercially using the cephalosporium mold.38
Many recent studies have established that the rate of cross-reactivity between penicillin and cephalosporins has been grossly overestimated. In fact, the rate of cross-reactivity between penicillin/amoxicillin and second- or third-generation cephalosporins is very low and may actually be lower than that between penicillins and other classes of antibiotics.44
The evidence for limited cross-reactivity. A summary of publications evaluating 38,846 children and adults with and without a history of penicillin allergy is presented in TABLE 1. The database included 2435 patients with a history of penicillin allergy and 961 patients with a history of penicillin allergy and positive skin-test results for penicillin or amoxicillin (total penicillin-allergic patients=3396). The allergic reaction rate is compared with 34,047 patients without a history of penicillin allergy and 1403 patients without a history of penicillin allergy and negative skin-test results for penicillin or amoxicillin (total penicillin-nonallergic patients=35,450).
When patients with a positive history of penicillin-allergy received first-generation cephalosporins, which share a chemical side chain similar to penicillin or amoxicillin (cephalothin, cephaloridine, cephalexin, cefadroxil, and cefazolin, plus the early second-generation cephalosporin, cefamandole), they exhibited a significant increased risk of an allergic reaction to the cephalosporin.
Second- and third-generation cephalosporins modified in size and the complexity of their side chains (eg, cefprozil, cefuroxime, ceftazidime, cefpodoxime, and ceftriaxone) were different enough from penicillin and ampicillin that they did not increase risk of allergic cross-reactivity (TABLE 1).

Many other studies have suggested that cross-reactive immune responses to cephalosporins depend on side chain structure;22,23,27,32,37,38,44-49 that is, cephalosporins with a 7-position side chain similar to benzylpenicillin are more likely to cross-react with penicillin (TABLE 2). Cephalosporins that share a similar 7-position or 3-position side chain are more likely to cross-react with each other.

Cephalosporin/penicillin cross-reactivity
Few studies have evaluated whether patients with primary hypersensitivity to cephalosporins will experience cross-reactivity with penicillin. Romano et al49 conducted skin tests and RASTs in patients with immediate allergic reactions to cephalosporins to examine responses to other cephalosporins and to classic penicillin determinants. About 1 in 5 patients allergic to a cephalosporin reacted to penicillin determinants, while most had positive results to other cephalosporins with the same or similar side-chains.
Limitations of skin testing
Penicillin skin testing in patients with a history of penicillin allergy does not reliably predict allergy to a cephalosporin unless the side chain of the penicillin or ampicillin reagent is similar to the cephalosporin side chain being tested.3 The positive and negative predictive values of skin testing results for cephalosporins are not well established; if the haptens that cause cephalosporin allergy were known, cross-reactivity with penicillins could be assessed directly. Cephalosporin skin testing works only for the specific drug and drugs with the same side chains, and can be done only if the drug is available in an IV or IM formulation.
Even a positive result does not guarantee a clinical reaction. When penicillin and cephalosporin skin tests or radioallergosorbent tests (RASTs) are positive, a clinical reaction is observed in only 10% to 60% of patients, depending on the reagent and study.50 For example, among 19 well-characterized patients allergic to penicillin who were studied for their sensitivity to the cephalosporins, cephaloridine and cefamandole (which have identical or very similar side chains to penicillin and were therefore potentially cross-reactive) only 2 (10.5%) reacted to cefamandole, while the other 17 patients tolerated both agents.26 In another study of clinical cross-reactivity between amoxicillin and cefadroxil in patients allergic to amoxicillin with good tolerance of penicillin, only 12% had an immediate allergic reaction to cefadroxil, despite the 2 drugs sharing an identical side chain.33 In a third study, allergenic cross-reactivity with cefadroxil and cefamandole was studied among 21 patients selectively allergic to amoxicillin; 8 (38%) had a positive response to cefadroxil (same side chain) and none to cefamandole (different side chain).32
Discussion
Sensible approach to penicillin-allergic patients
Question patients who report penicillin allergy. In many cases, penicillin may not actually have been taken, or patients may have had non-immunologic adverse events such as vomiting, diarrhea, or nonspecific rash; toxic effects; or contemporaneous side effects inappropriately attributed to the drug. These patients can receive penicillin, amoxicillin, or the cephalosporins.
Without the ability to detect patients with IgE antibody to penicillin prospectively or to distinguish true IgE immunologic reactions from idiopathic reactions in patients receiving cephalosporins, it is impossible to definitely claim that increased immune or IgE-mediated reactions to cephalosporins occur in true penicillinallergic (IgE) patients.
When a cephalosporin is/is not safe for a penicillin-allergic patient. Only IgE-mediated reactions—such as anaphylaxis or hypotension, laryngeal edema, wheezing, angioedema, or urticaria—are likely to become more severe with time. Therefore, with a patient who has had a true IgE-mediated reaction to a penicillin, avoid using cephalosporins with a similar side chain. You may, however, give cephalosporins that have different side chains. Cephalosporins may also be used for patients who have had non-IgE-mediated adverse reactions (“non-type I allergy”)21 to a penicillin, such as a non-pruritic, non-urticarial morbilliform or maculopapular rash.
How prevalent is primary cephalosporin allergy? Even if the patient is not allergic to penicillin, cephalosporins can cause allergic or immune-mediated reactions in approximately 1% to 3% of patients. A patient who had an allergic reaction to a specific cephalosporin probably should not receive that cephalosporin again. The risk of a reaction with a different cephalosporin is very low to nonexistent if the side chains of the 2 drugs are dissimilar.
Bottom line. Penicillin-allergic patients have indeed shown an increased incidence of allergic reactions to cephalothin, cephaloridine, cephalexin, cefadroxil, cefazolin, and cefamandole. However, the risk has been overestimated because most studies reporting this cross-reactivity were flawed (because penicillins were contaminated with cephalosporins) and then failed to account for the fact that penicillin-allergic patients have a 3-fold increased risk of allergic reactions even to nonrelated drugs.51
For patients truly allergic to penicillin, the risk of a reaction from a cephalosporin with side chains that differ from penicillin/amoxicillin (cefuroxime, cefpodoxime, cefdinir, and ceftriaxone, as endorsed by the AAFP) is so low that use is justified and medico-legally defensible by the currently available evidence.
CORRESPONDENCE
Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Avenue, Box 672, Rochester, New York 14642. E-mail: [email protected]
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.
1. Levine B. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966;275:1115-1125.
2. Torres M, Gonzales F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. Int Arch Allergy Immunol 1997;113:342-344.
3. Pichichero M. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115:1048-1057.
4. Arndt J, Jick H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. JAMA 1976;235:918-923.
5. Platt R. Adverse effects of third-generation cephalosporins. J Antimicrob Chemother 1982;10(C):135-140.
6. Sanders CV, Greenberg RN, Marier RL. Cefamandole and cefoxitin. Ann Intern Med 1985;103:70-78.
7. Levine LR. Quantitative comparison of adverse reactions to cefaclor vs. amoxicillin in a surveillance study. Pediatr Infect Dis 1985;4:358-361.
8. Norrby S. Side effects of cephalosporins. Drug 1987;34(Suppl 2):105-120.
9. Idsoe O, Guthe T, Wilcox R. Nature and extent of penicillin side-reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-188.
10. Petz L. Immunologic reactions of humans to cephalosporins. Post Grad Med J 1971;47(Suppl):64-69.
11. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-2463.
12. Walter E, Moelling K, Pavlovic J, et al. Micro-encapsulation of DNA using poly (DL-lactide-co-glycolide): stability issues and release characteristics. J Controlled Release 1999;61:361-374.
13. Weinstein L, Kaplan K, Chang T. Treatment of infections in man with cephalothin. JAMA 1964;189:829-834.
14. Griffith R, Black H. Cephalothin—a new antibiotic. Preliminary clinical and laboratory studies. JAMA 1964;189:823-828.
15. Apicella M, Perkins R, Salsaw S. Cephaloridine treatment of bacterial infections. Am J Med Sci 1966;251:266-276.
16. Assem E, Vickers M. Tests for penicillin allergy in man: The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-269.
17. Marks J, Garrett R. Cephalexin in general practice. Post Grad Med J 1970;46(Suppl):113-117.
18. Stewart G. Cross-allergenicity of penicillin G and related substances. Lancet 1962;1:509-510.
19. Dash C. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975;(1 Suppl):107-118.
20. Petz L. Immunologic cross-reactivity between penicillins and cephalosporins. J Infect Dis 1978;137:S74-S79.
21. Thoburn R, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966;198:345-348.
22. Solley G, Gleich G, Van Dellen R. Penicillin allergy: Clincal experience with a battery of skin test reagents. J Allergy Clin Immunol 1982;69:238-244.
23. Warrington R, McPhillipps S. Independent anaphylaxis to cefazolin without allergy to other beta-lactam antibiotics. J Allergy Clin Immunol 1996;98:460-462.
24. Sullivan T, Wedner H, Shatz G, et al. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-180.
25. Saxon A. Immediate hypersensitivity reactions to b-lactam antibiotics. Rev Infect Dis 1983;5(Suppl 2):S368-S378.
26. Blanca M, Fernandez J, Miranda A, et al. Cross reactivity between penicillins and cephalosporins: Clinical and immunological studies. J Allergy Clin Immunol 1989;83:381-385.
27. Lin R. A perspective on penicillin allergy. Arch Intern Med 1992;152:930-937.
28. Martin J, Igea J, Fraj J, et al. Allergy to amoxicillin in patients who tolerated benzylpenicillin, aztreonam, and ceftazidime. Clin Infect Dis 1992;14:592-593.
29. Shepherd G, Burton D. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy [abstract]. J Allergy Clin Immunol 1993;91:262.-
30. Audicana M, Bernaola G, Urrutia I, et al. Allergic reactions to betalactams: Studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporins. Allergy 1994;49:108-113.
31. Anne S, Reisman R. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Annals Allergy Asthma Immunology 1995;74:167-170.
32. Miranda A, Blanca M, Vega J, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol 1996;98:671-677.
33. Sastre J, Quijano L, Novalbos A, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996;51:383-386.
34. Pichichero M, Pichichero D. Selecting skin testing reagents to predict amoxicillin and cephalosporin allergy. Pediatr Asthma Allergy Immunol 1997;11:79-93.
35. Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy 2001;31:438-443.
36. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med 2004;141:16-22.
37. Torres M, Blanca M, Garcia J. Evaluation of a large cohort of subjects allergic to penicillins [abstract]. J Allergy Clin Immunol 1995;95:285.-
38. Kelkar P, Li J. Cephalosporin allergy. N Engl J Med 2001;385:804-809.
39. Kabins S, Einstein B, Cohen S. Anaphylactic reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165.-
40. Romano A, Piunti E, De Fronso M, et al. Selective immediate hypersensitivity to ceftriaxone. Allergy 2000;55:418-419.
41. Pumphrey R, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-1158.
42. Mayorga C, Torres M, Blanca M. Cephalosporin allergy. N Engl J Med 2002;236:380-381.
43. Mayorga C, Ovispo T, Jimeno L. Epitope mapping of betalactam antibiotics with the use of monoclonal antibodies. Toxicology 1995;97:225-34.
44. Weiss M, Adkinson N. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1998;18:515-540.
45. Blaiss M, DeShazo R. Drug allergy. Pediatr Clin North Am 1998;35:1131-1147.
46. Baumgart K, Baldo B. Cephalosporin allergy. N Engl J Med 2002;346:380.-
47. James J. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. Pediatrics 1999;104:367.-
48. Pichichero M, Pichichero D. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-143.
49. Romano A, Mayorga C, Torres M, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-1183.
50. Salkind A, Cuddy P, Foxworth J. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001;285:2498-2505.
51. Smith J, Johnson J, Cluff L. Studies on the epidemiology of adverse drug reactions: II. An evaluation of penicillin allergy. N Engl J Med 1966;274:998-1002.
52. Warrington R, Simons F, Ho H, et al. Diagnosis of penicillin allergy by skin testing: the Manitoba experience. Can Med Assoc J 1978;118:797-791.
53. Saxon A, Beall G, Rohr A. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-215.