User login
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).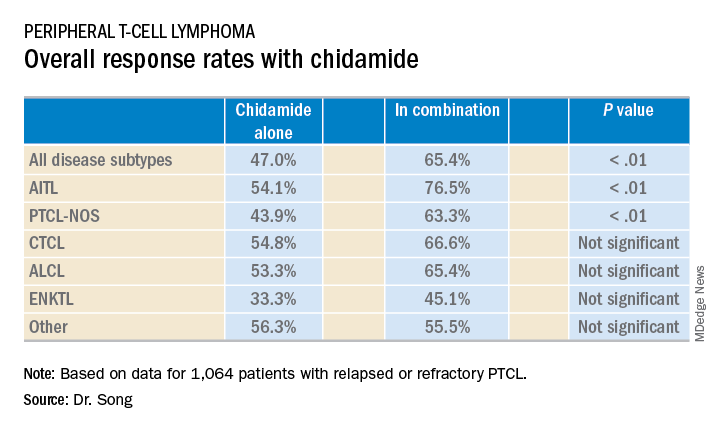
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Real-world data suggest chidamide may be more effective against relapsed or refractory peripheral T-cell lymphoma (PTCL) than a pivotal study indicated.
Single-agent chidamide produced an overall response rate of 47.0% in a real-world study of more than 1,000 patients, compared with the 28.0% overall response rate that was observed in the phase 2 study of chidamide (Ann Oncol. 2015 Aug;26[8]:1766-71).
Yuqin Song, MD, PhD, of Peking University Cancer Hospital and Institute in Beijing, China, presented data from the real-world study at the annual T-cell Lymphoma Forum.
Dr. Song said this study is the largest cohort of real-world patients with relapsed or refractory PTCL. She and her colleagues analyzed data on 1,064 patients treated at 216 sites across China between February 2015 and December 2017.
The patients had a median age of 54 years, 63.9% were male, and 88.1% had stage III-IV disease.
Disease subtypes included PTCL not otherwise specified (NOS, 38.0%), angioimmunoblastic T-cell lymphoma (AITL, 29.1%), extranodal natural killer T-cell lymphoma (ENKTL, 13.4%), anaplastic large-cell lymphoma (ALCL, 9.1%), and others (10.3%), including cutaneous T-cell lymphoma (CTCL).
Fifty-two percent of patients (n = 553) received chidamide as a single agent, and 48% (n = 511) received the drug with other agents. The most common treatment regimens combined with chidamide were the following
- Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP, 20.7%).
- Gemcitabine, dexamethasone, and cisplatin (GDP, 11.8%).
- Etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH, 9.8%).
- Patients with ENKTL received chidamide with L-asparaginase (35.4%) or without it (64.5%).
The median follow-up was 4.9 months (range, 0-36.2 months). Across disease subtypes, the overall response rate was 47.0% with single-agent chidamide and 65.4% when chidamide was given in combination with other agents (P less than .01).
The median overall survival was 400 days for all patients, 342 days for patients treated with chidamide alone, and 457 days for patients who received combination therapy. The 1-year overall survival rates were 52%, 48%, and 56%, respectively.
Dr. Song said these data verify the efficacy of chidamide as a single agent and suggest chidamide might lead to improved survival in refractory or relapsed PTCLs.
Chidamide was generally well tolerated in this study, Dr. Song said. There were no unexpected adverse events (AEs) and most were grade 1 or 2.
The most common AEs (of any grade) observed with single-agent chidamide were neutropenia (42.9%), thrombocytopenia (40.5%), fatigue (38.3%), anemia (31.6%), and nausea/vomiting (21.0%).
The most common AEs observed with chidamide in combination were neutropenia (61.4%), thrombocytopenia (58.5%), fatigue (56.2%), anemia (54.2%), nausea/vomiting (30.7%), and fever (22.1%).
This study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Single-agent chidamide had an overall response rate of 47.0% among relapsed/refractory PTCL patients, compared with 65.4% when used in combination with other agents (P less than .01).
Study details: A real-world cohort of 1,064 relapsed/refractory PTCL patients treated at 216 sites across China between February 2015 and December 2017.
Disclosures: The study was supported by the Union for China Lymphoma Investigators and the Chinese Society of Clinical Oncology. Dr. Song did not disclose any conflicts of interest.

