User login
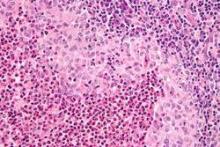
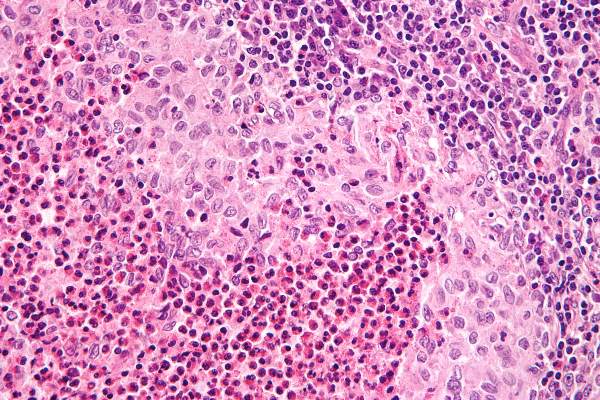
After standard therapy failed, cladribine plus cytosine-arabinoside (cytarabine [Ara-C]) was effective in treating patients with high-risk, organ-positive Langerhans cell histiocytosis; however, all patients experienced hematologic toxicity, based on the results of a phase II study.
The overall response rate was 92% and the 5-year survival rate was 85% (95% confidence interval, 65%-94%). The study included 27 patients, median age 0.73 years (range, 0.1-3.3 years), who did not respond to standard therapy and had involvement of at least one risk organ (spleen, liver, or bone marrow), reported Dr. Jean Donadieu of Service d’Hemato-Oncologie Pediatrique, Hopital Trousseau, Paris, and colleagues (Blood. 2015;126[12]:1415-23).
The study had strict inclusion criteria, and treatment was only given to children with severe Langerhans cell histiocytosis (LCH), which historically has had long-term survival rates of 20% to 30%.
After one course of combination therapy, all of the patients experienced pancytopenia (grade 4, median duration 23 days) complicated by fever, and 30% had severe infections. In the two patients who died as a result of protocol-related toxicity, the cumulative doses of cladribine were 250 mg/m2 and 225 mg/m2.
Given that cladribine is known to cause severe lymphopenia and prolonged pancytopenia, the researchers suggested limiting the cumulative dose to 200 mg/m2 and limiting cladribine/Ara-C treatment to a maximum of three courses. The researchers further cautioned that the treatment should be performed at institutions trained in providing acute myeloid leukemia chemotherapy regimens or hematopoietic transplantation.
The response to cladribine/Ara-C treatment was slow in LCH patients. Those who responded to the therapy, but had not achieved a complete response after two to three courses, achieved a complete response after continuation with a less toxic therapy.
“Accordingly, we recommend that care be taken at this time to avoid potential toxicity on the one hand but also, on the other hand, to avoid prematurely deciding that the therapy is not working and possibly discontinuing treatment that might help longer-term,” wrote Dr. Donadieu and associates.
A comprehensive LCH activity scoring system determined the initial severity of disease and assessed treatment efficacy. With successful therapy, the median disease activity score decreased from 12 (range, 6-20) to 3 (range, 0-18; P less than .0001).
Dr. Donadieu and coauthors reported having no disclosures.
In previous collaborative trials, patients with the worst prognosis (overall 2-year survival of less than 30%) had disease involving the bone marrow, liver, or spleen; did not respond to routine induction treatment; or had reactivation in one of the high-risk organs. The bottom line in this report is the overall response rate of 92% and long-term survival rate of 85% among 27 patients with severe LCH.
This report demonstrates that children who have disease of the bone marrow, liver, or spleen and who do not respond to standard induction therapy can be treated with intensive chemotherapy.
The next step is the LCH-IV study, launched by the Histiocyte Society to test a slightly modified treatment plan that attempts to decrease the toxicity yet maintain the improved overall survival seen in this phase II trial.
Dr. Kimo C. Stine is a pediatric hematologist-oncologist at Arkansas Children’s Hospital, Little Rock, and professor of pediatrics at the University of Arkansas for Medical Sciences. These remarks were part of an editorial (Blood. 2015;126[12]:1399-1400) accompanying the report. Dr. Stine had no disclosures to report.
In previous collaborative trials, patients with the worst prognosis (overall 2-year survival of less than 30%) had disease involving the bone marrow, liver, or spleen; did not respond to routine induction treatment; or had reactivation in one of the high-risk organs. The bottom line in this report is the overall response rate of 92% and long-term survival rate of 85% among 27 patients with severe LCH.
This report demonstrates that children who have disease of the bone marrow, liver, or spleen and who do not respond to standard induction therapy can be treated with intensive chemotherapy.
The next step is the LCH-IV study, launched by the Histiocyte Society to test a slightly modified treatment plan that attempts to decrease the toxicity yet maintain the improved overall survival seen in this phase II trial.
Dr. Kimo C. Stine is a pediatric hematologist-oncologist at Arkansas Children’s Hospital, Little Rock, and professor of pediatrics at the University of Arkansas for Medical Sciences. These remarks were part of an editorial (Blood. 2015;126[12]:1399-1400) accompanying the report. Dr. Stine had no disclosures to report.
In previous collaborative trials, patients with the worst prognosis (overall 2-year survival of less than 30%) had disease involving the bone marrow, liver, or spleen; did not respond to routine induction treatment; or had reactivation in one of the high-risk organs. The bottom line in this report is the overall response rate of 92% and long-term survival rate of 85% among 27 patients with severe LCH.
This report demonstrates that children who have disease of the bone marrow, liver, or spleen and who do not respond to standard induction therapy can be treated with intensive chemotherapy.
The next step is the LCH-IV study, launched by the Histiocyte Society to test a slightly modified treatment plan that attempts to decrease the toxicity yet maintain the improved overall survival seen in this phase II trial.
Dr. Kimo C. Stine is a pediatric hematologist-oncologist at Arkansas Children’s Hospital, Little Rock, and professor of pediatrics at the University of Arkansas for Medical Sciences. These remarks were part of an editorial (Blood. 2015;126[12]:1399-1400) accompanying the report. Dr. Stine had no disclosures to report.
After standard therapy failed, cladribine plus cytosine-arabinoside (cytarabine [Ara-C]) was effective in treating patients with high-risk, organ-positive Langerhans cell histiocytosis; however, all patients experienced hematologic toxicity, based on the results of a phase II study.
The overall response rate was 92% and the 5-year survival rate was 85% (95% confidence interval, 65%-94%). The study included 27 patients, median age 0.73 years (range, 0.1-3.3 years), who did not respond to standard therapy and had involvement of at least one risk organ (spleen, liver, or bone marrow), reported Dr. Jean Donadieu of Service d’Hemato-Oncologie Pediatrique, Hopital Trousseau, Paris, and colleagues (Blood. 2015;126[12]:1415-23).
The study had strict inclusion criteria, and treatment was only given to children with severe Langerhans cell histiocytosis (LCH), which historically has had long-term survival rates of 20% to 30%.
After one course of combination therapy, all of the patients experienced pancytopenia (grade 4, median duration 23 days) complicated by fever, and 30% had severe infections. In the two patients who died as a result of protocol-related toxicity, the cumulative doses of cladribine were 250 mg/m2 and 225 mg/m2.
Given that cladribine is known to cause severe lymphopenia and prolonged pancytopenia, the researchers suggested limiting the cumulative dose to 200 mg/m2 and limiting cladribine/Ara-C treatment to a maximum of three courses. The researchers further cautioned that the treatment should be performed at institutions trained in providing acute myeloid leukemia chemotherapy regimens or hematopoietic transplantation.
The response to cladribine/Ara-C treatment was slow in LCH patients. Those who responded to the therapy, but had not achieved a complete response after two to three courses, achieved a complete response after continuation with a less toxic therapy.
“Accordingly, we recommend that care be taken at this time to avoid potential toxicity on the one hand but also, on the other hand, to avoid prematurely deciding that the therapy is not working and possibly discontinuing treatment that might help longer-term,” wrote Dr. Donadieu and associates.
A comprehensive LCH activity scoring system determined the initial severity of disease and assessed treatment efficacy. With successful therapy, the median disease activity score decreased from 12 (range, 6-20) to 3 (range, 0-18; P less than .0001).
Dr. Donadieu and coauthors reported having no disclosures.
After standard therapy failed, cladribine plus cytosine-arabinoside (cytarabine [Ara-C]) was effective in treating patients with high-risk, organ-positive Langerhans cell histiocytosis; however, all patients experienced hematologic toxicity, based on the results of a phase II study.
The overall response rate was 92% and the 5-year survival rate was 85% (95% confidence interval, 65%-94%). The study included 27 patients, median age 0.73 years (range, 0.1-3.3 years), who did not respond to standard therapy and had involvement of at least one risk organ (spleen, liver, or bone marrow), reported Dr. Jean Donadieu of Service d’Hemato-Oncologie Pediatrique, Hopital Trousseau, Paris, and colleagues (Blood. 2015;126[12]:1415-23).
The study had strict inclusion criteria, and treatment was only given to children with severe Langerhans cell histiocytosis (LCH), which historically has had long-term survival rates of 20% to 30%.
After one course of combination therapy, all of the patients experienced pancytopenia (grade 4, median duration 23 days) complicated by fever, and 30% had severe infections. In the two patients who died as a result of protocol-related toxicity, the cumulative doses of cladribine were 250 mg/m2 and 225 mg/m2.
Given that cladribine is known to cause severe lymphopenia and prolonged pancytopenia, the researchers suggested limiting the cumulative dose to 200 mg/m2 and limiting cladribine/Ara-C treatment to a maximum of three courses. The researchers further cautioned that the treatment should be performed at institutions trained in providing acute myeloid leukemia chemotherapy regimens or hematopoietic transplantation.
The response to cladribine/Ara-C treatment was slow in LCH patients. Those who responded to the therapy, but had not achieved a complete response after two to three courses, achieved a complete response after continuation with a less toxic therapy.
“Accordingly, we recommend that care be taken at this time to avoid potential toxicity on the one hand but also, on the other hand, to avoid prematurely deciding that the therapy is not working and possibly discontinuing treatment that might help longer-term,” wrote Dr. Donadieu and associates.
A comprehensive LCH activity scoring system determined the initial severity of disease and assessed treatment efficacy. With successful therapy, the median disease activity score decreased from 12 (range, 6-20) to 3 (range, 0-18; P less than .0001).
Dr. Donadieu and coauthors reported having no disclosures.
FROM BLOOD
Key clinical point: In children with high-risk, refractory Langerhans cell histiocytosis (LCH), treatment with cladribine plus cytarabine was effective but highly toxic.
Major finding: The overall response rate was 92%, and the 5-year survival rate was 85% (95% CI, 65%-94%).
Data source: The phase II study included 27 patients (mean age, 0.73 years) who had involvement of at least one risk organ (spleen, liver, bone marrow) and had failed standard therapy.
Disclosures: Dr. Donadieu and coauthors reported having no disclosures.