User login
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
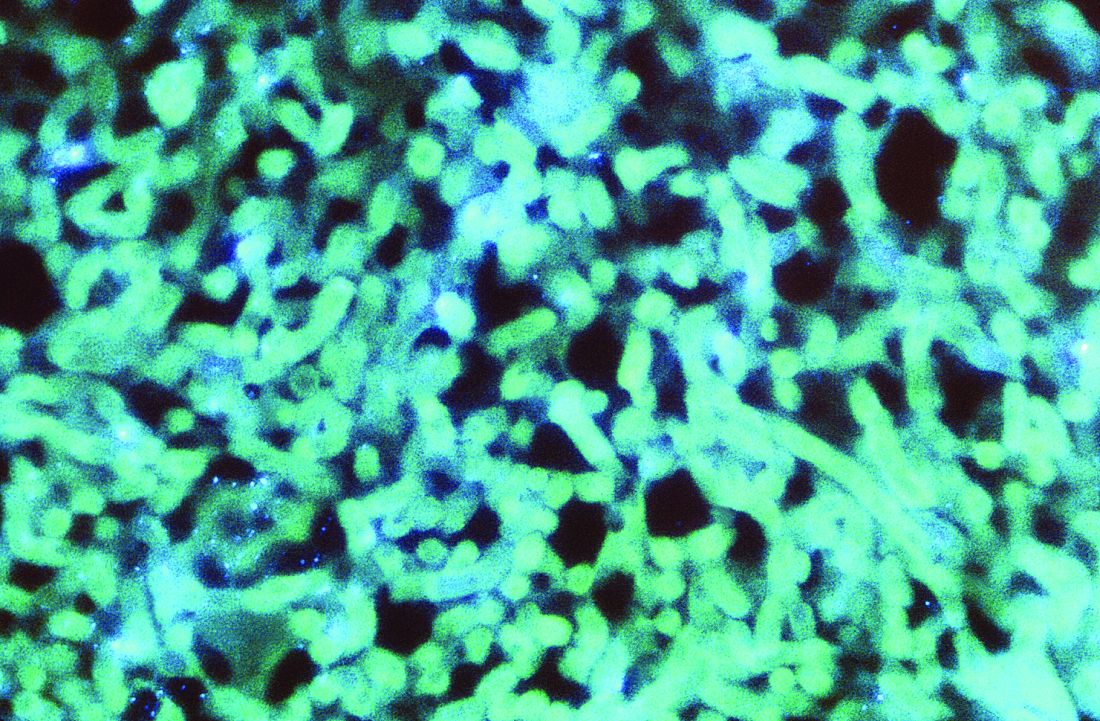
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
FROM CLINICAL INFECTIOUS DISEASES