User login
Latest COVID-19 Shot May Cut Severe Outcomes in Veterans
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID Linked to Eye Issues, But Vaccine Offers Protection
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with COVID had a higher risk of developing diplopia and cranial nerve VI palsy than those with influenza. Compared with unvaccinated patients, recipients of mRNA vaccines against SARS-CoV-2 had a more than 30% reduced risk of developing posterior-segment complications including retinal edema, vitreous hemorrhage, and optic neuritis.
METHODOLOGY:
- Researchers conducted a retrospective cohort analysis of US electronic health records from March 2020 to April 2021 to assess eye complications after COVID and the effect of mRNA vaccination on them.
- They analyzed matched cohorts of 73,654 vaccinated patients with COVID (mean age, 60.6 years; 61.6% women) and 73,654 unvaccinated patients with the condition (mean age, 61.2 years; 62.8% women); vaccination status was determined based on recorded receipt of an mRNA vaccine.
- In a separate matched analysis, 77,809 patients with COVID (mean age, 39.3 years; 58.8% women) were compared with a historic cohort of 77,809 patients with influenza (mean age, 39.7 years; 58.9% women).
- The incidence of ophthalmic conditions — retinal artery occlusion, retinal vein occlusion, retinal edema, vitreous hemorrhage, and neuro-ophthalmic manifestations — was assessed within 4 months of infection.
TAKEAWAY:
- Vaccinated patients with COVID had 32% lower odds of retinal edema (odds ratio [OR], 0.68; 99.5% CI, 0.54-0.85), 45% lower odds of vitreous hemorrhage (OR, 0.55; 99.5% CI, 0.44-0.68), and 40% lower odds of optic neuritis (OR, 0.60; 99.5% CI, 0.43-0.85) than unvaccinated patients with the disease.
- No significant differences were found in the incidence of retinal artery occlusion, retinal vein occlusion, or retinal hemorrhage between the vaccinated and unvaccinated cohorts.
- Patients with COVID had markedly higher odds of diplopia (OR, 1.89; 99.5% CI, 1.53-2.32) and cranial nerve VI palsy (OR, 3.19; 99.5% CI, 1.82-5.59) than those with influenza.
- The incidence of other neuro-ophthalmic manifestations and retinal complications was similar between patients with COVID and those with influenza.
IN PRACTICE:
“The complications we assessed were rare, though our results showed an increased incidence of retinal edema, vitreous hemorrhage, and optic neuritis in the nonvaccinated COVID-19 cohort,” the researchers reported.
“The increased incidence of retinal edema and vitreous hemorrhage in the nonvaccinated cohort suggests a potential for COVID-19 to affect posterior segment structures,” they added.
SOURCE:
This study was led by Alexander E. Azar, Case Western Reserve University School of Medicine, Cleveland. It was published online in Eye.
LIMITATIONS:
This study could not determine if vaccination against COVID could prevent ophthalmic manifestations. Vaccination status may have been underreported since many participants received COVID vaccines at pharmacies or community centers not directly documented in the electronic health records. The study’s timeframe only reflected data from early strains of SARS-CoV-2 between March 2020 and April 2021, potentially limiting generalizability to newer variants or later vaccination phases.
DISCLOSURES:
This study received support from the Clinical and Translational Science Collaborative of Cleveland, funded by the National Institutes of Health, National Center for Advancing Translational Science, and other sources. Some authors reported serving as consultants, participating in speakers’ bureaus, receiving personal fees, and having other ties with multiple pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
US Health Official Calls for Separating Measles Combination Shots, Pulls Broad COVID Vaccine Support
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
(Reuters) -A top U.S. health official on Monday called for the combined measles-mumps-rubella shot to be broken up, drawing a quick rebuke from vaccine maker Merck, which said there is no scientific evidence that shows any benefit to doing so.
The U.S. CDC earlier on Monday pulled broad support for COVID-19 shots, saying they should be administered through shared decision-making with a health care provider in accordance with recommendations from Health Secretary Robert F. Kennedy Jr.’s hand-picked vaccine advisory panel.
The acting director of the Centers for Disease Control and Prevention, Jim O’Neill, in an X post on Monday called on vaccine manufacturers to develop three separate vaccines to replace the combined MMR inoculation.
In a September 23 news conference at the White House, President Donald Trump delivered medical advice to pregnant women and parents of young children, repeatedly telling them common vaccines should not be taken together or so early in a child’s life, and urging them not to use or administer Tylenol, against the advice of medical societies.
Kennedy, a long-time anti-vaccine crusader before taking on the nation’s top health post, has linked vaccines to autism and sought to rewrite the country’s immunization policies. He fired all members of the national vaccine advisory board of outside experts and replaced them with new members, many of whom shared his views. The committee is reviewing the childhood vaccine schedule.
The causes of autism are unclear. But no rigorous studies have found links between autism and vaccines or medications, or their components such as thimerosal or formaldehyde. Vaccination rates have declined as autism rates have climbed.
MERCK, EXPERTS DEFEND MMR SHOT
Merck said there is no published scientific evidence that shows any benefit in separating the MMR shot.
According to the U.S. Food and Drug Administration’s website, there are currently no separate single virus shots for measles, mumps or rubella licensed for use in the United States. That means manufacturers could need to go through the FDA approval process before any become available.
“Use of the individual components of combination vaccines increases the number of injections for the individual and may result in delayed or missed immunizations,” Merck said in a statement.
Dr. Rana Alissa, president of the Florida chapter of the American Academy of Pediatrics, said the purpose of combining the three shots in the MMR vaccine is not only to save parents extra visits to the doctor’s office.
“Studies have shown that when you give them together, the immune response is much better,” she said. “This is how you get lifelong immunity.”
GSK, which also makes an MMR shot, declined to comment. A spokesman for the U.S. Department of Health and Human Services, where O’Neill is deputy secretary, was not immediately available for comment.
The break-up of the MMR shot would “falsely imply that there is something unsafe about giving the measles, mumps, and rubella vaccines at the same time,” said Dr. Amesh Adalja, an infectious disease expert at the Johns Hopkins Center for Health Security.
“It would be another example of the federal government pandering to the anti-vaccine movement,” Adalja added.
Earlier in the day, the CDC signed off on the advisers’ recommendations against use of the combined measles-mumps-rubella-varicella vaccine before the age of 4 years because of a slight risk of seizures related to high fevers. Instead, varicella, commonly known as chickenpox, is recommended as a standalone shot.
Merck also makes the measles-mumps-rubella-varicella shot.
CDC CHANGES COVID VIEWS
The new CDC recommendation on the COVID vaccine calls for physician involvement but maintains access for the shot through health insurance.
The immunization schedules will be updated on the CDC website by Tuesday, the agency said.
The recommendations come after upheaval at the CDC, including the ouster of its former Director Susan Monarez, who had resisted changes to vaccine policy advanced by Kennedy. Monarez said she was told to rubber-stamp the committee’s recommendations without reviewing the scientific evidence.
The new advisory panel made its recommendations at a two-day meeting in September that highlighted deep divisions over the future of the U.S. immunization schedules under Kennedy.
The American Academy of Pediatrics, an influential U.S. medical group, has already broken from federal policy and pushed its own vaccine recommendations, suggesting all young children get vaccinated against COVID-19.
The U.S. Food and Drug Administration in August cleared updated COVID-19 vaccines for everyone over age 65, but limited its approval for younger people to those with health risks.
The 3 approved COVID shots are made by Pfizer with German partner BioNTech, Moderna, and Novavax with Sanofi.
(Reporting by Mariam Sunny in Bengaluru, Michael Erman in New York and Julie Steenhuysen in Chicago; Editing by Caroline Humer and Bill Berkrot)■
A version of this article appeared on Medscape.com.
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).
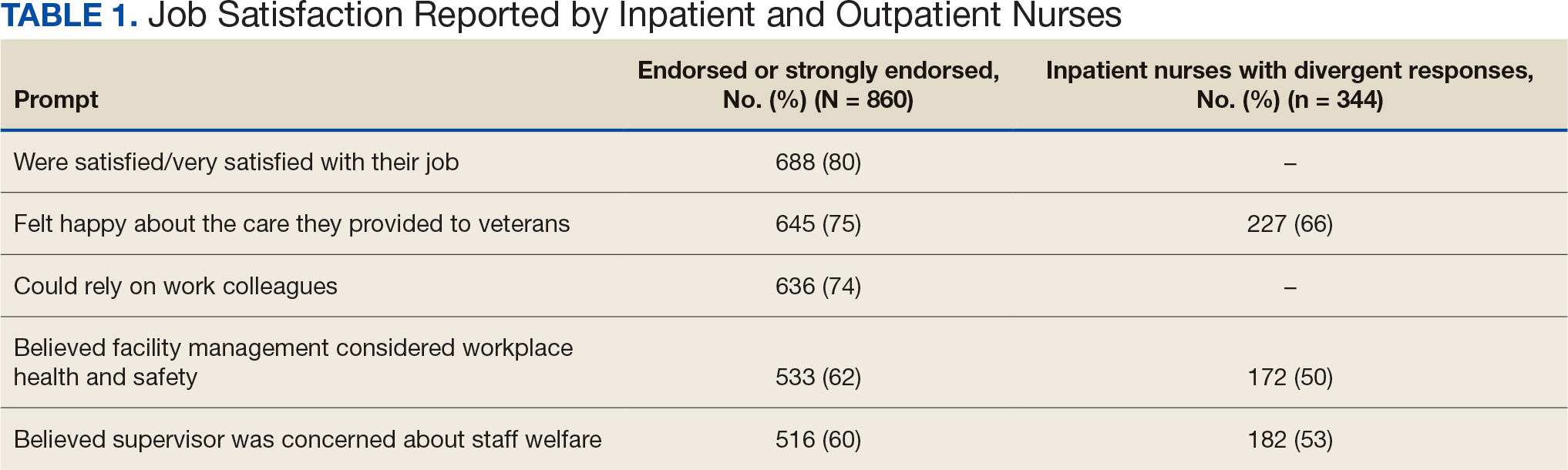
Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).
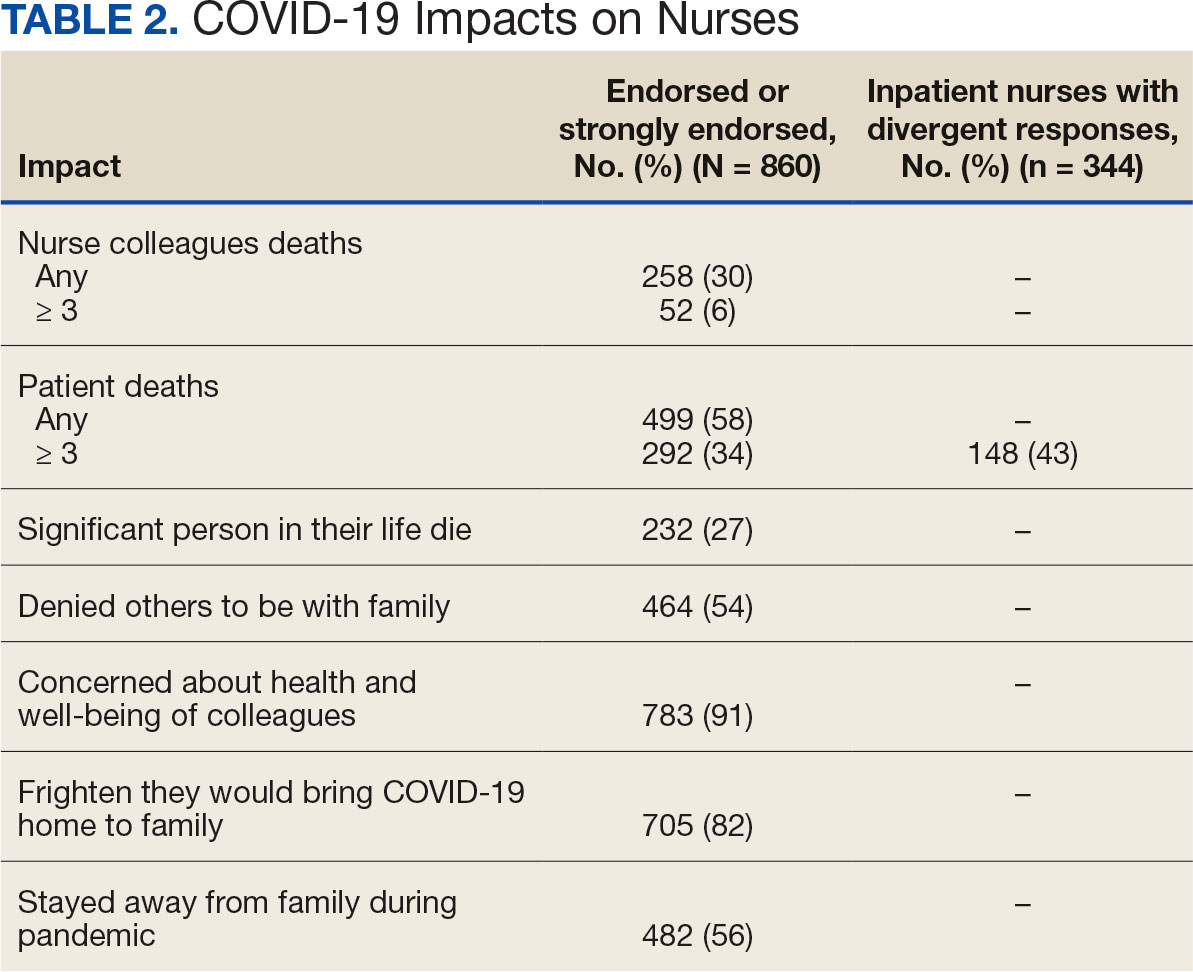
A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).
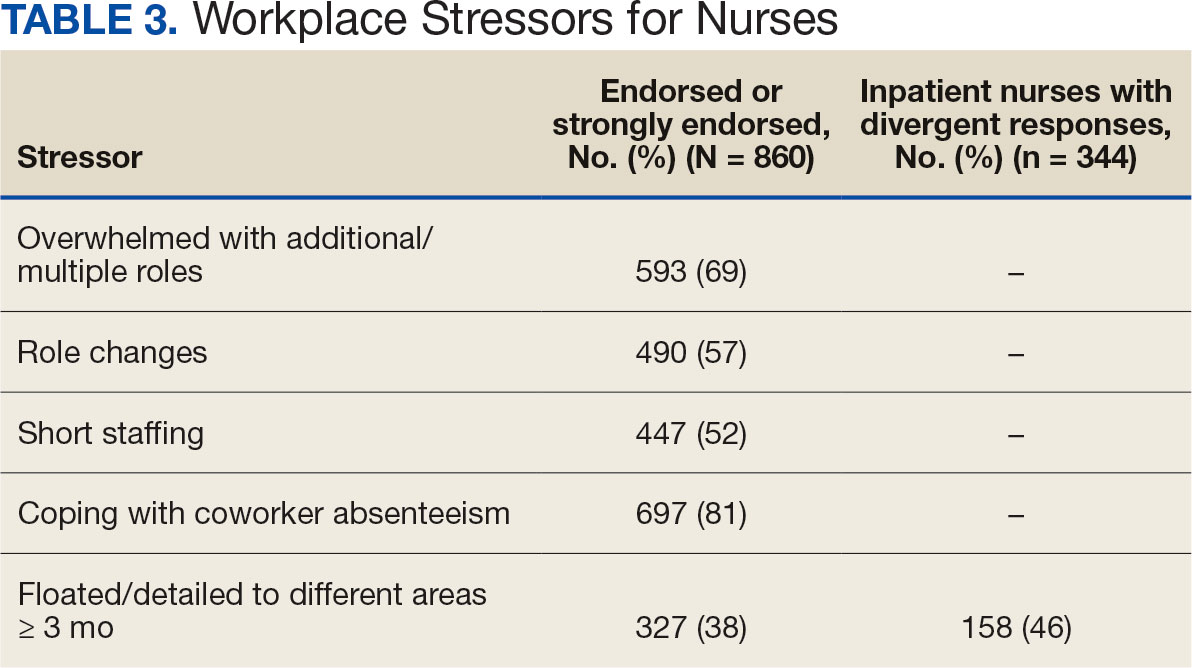
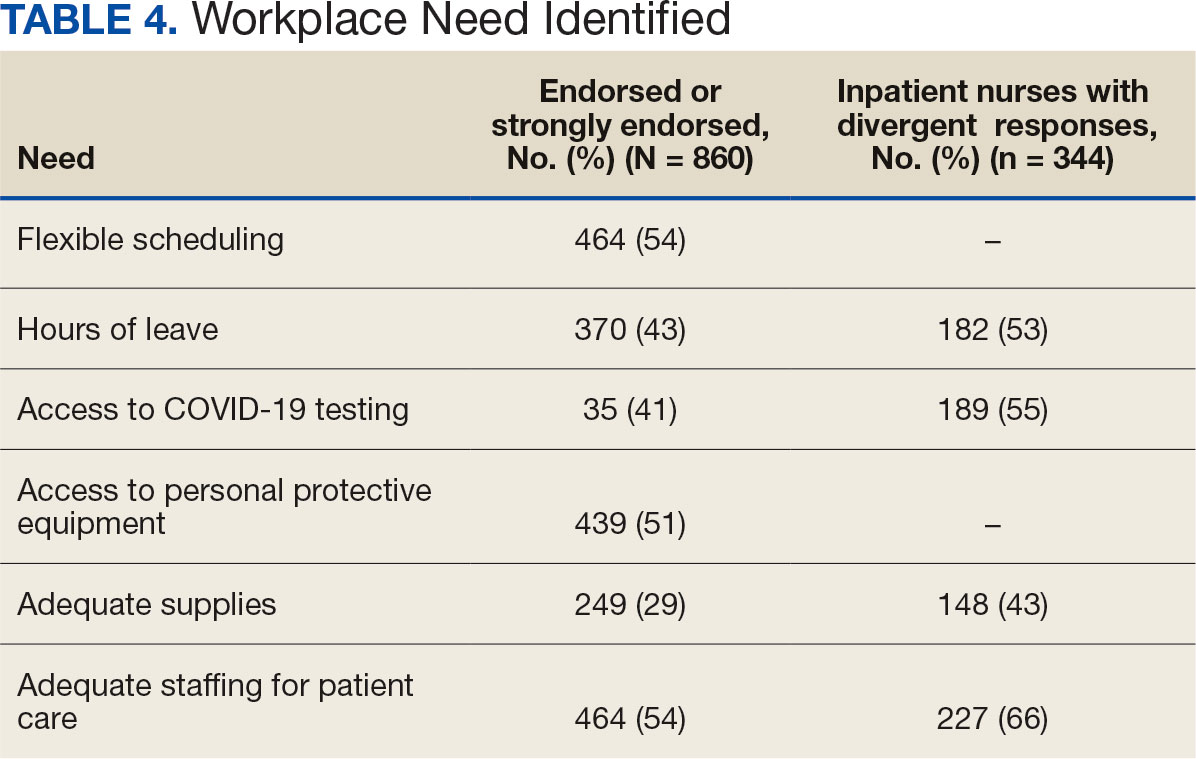
Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).
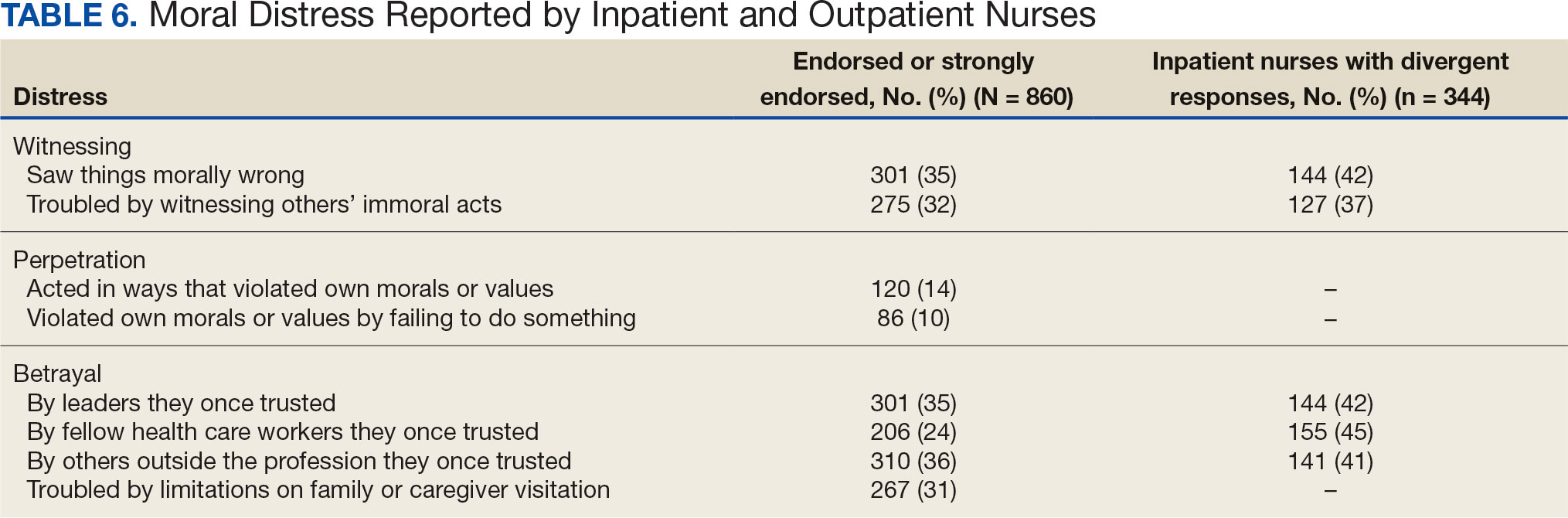
Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.


DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
Most Kids With COVID-Linked MIS-C Recover by 6 Months
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Reducing Risk, One Mask at a Time: What the Science Says
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
A few items bring back unpleasant memories of COVID-19, such as masks. However, they are among the simplest and most effective ways to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). If everyone had worn them correctly, the transmission could have been reduced as much as ninefold, according to a theoretical study published in Physical Review E by Richard P. Sear, PhD, from the University of Surrey, Guildford, England.
Study Overcomes Limitations
This study aimed to address the limitations of epidemiological investigations of masks, which can be complex and error-prone. Sear used data obtained from the UK’s COVID-19 app, totaling 7 million contacts, to create a mathematical model of virus transmission, focusing on the correlation between contact duration and infection. The model estimates that if all UK residents had worn masks during every potential exposure, virus transmission would have been approximately nine times lower.
Although this is a mathematical model, it adds to the growing evidence that supports the benefits of masks. Masks are among the best strategies for treating SARS-CoV-2. This conclusion has been supported by several systematic reviews and additional statistical studies. Conversely, the decision to relax and eliminate mask regulations has had consequences that have received little attention.
As expected, removing the mask mandate leads to increased virus transmission, resulting in more hospitalizations and deaths. A 2024 study estimated that in Japan, where cultural factors lead to much higher mask use in public than in Europe, the decline in mask use from 97% of the population in 2022 to 63% in October 2023 may have caused an additional 3500 deaths.
Impact Beyond SARS-CoV-2
One remarkable effect of non-pharmaceutical interventions during the pandemic was the probable extinction of an entire influenza strain (B/Yamagata), which could improve future influenza vaccines and significantly reduce the spread of respiratory syncytial virus. While this was not solely caused by masks, it was also influenced by emergency measures such as lockdowns and social distancing. These behavioral changes can positively alter the landscape of infectious diseases.
Masks play a role in reducing influenza transmission during pandemics. Their effectiveness has been supported by several studies and systematic reviews on a wide range of respiratory viruses. A randomized clinical trial involving 4647 Norwegian participants from February to April 2023, published in May 2024 by the British Medical Journal, suggested that wearing a mask reduces the incidence of respiratory symptoms. Specifically, 8.9% of those who wore masks reported respiratory symptoms during the study period compared with 12.2% of those who did not, representing a relative risk reduction of 27%.
Widespread mask use could also protect against other factors such as fine particulate matter, indirectly reducing the risk for various health conditions. A retrospective study involving 7.8 million residents in the Chinese city of Weifang, published in December 2024 by BMC Public Health, suggested that mask use during the pandemic may have also protected the population from pollution, reducing the number of stroke cases by 38.6% over 33 months of follow-up.
Although there are still voices in bioethics calling for the reintroduction of mask mandates in public places, it is unlikely that, barring emergencies, mask mandates are politically and socially acceptable today. Mask use is also considered a politically polarizing topic in several Western countries. Nevertheless, it is worth considering whether, as we move away from the acute phase of the COVID-19 pandemic, we can more objectively promote the use of masks in public places.
Communicating the importance of public health initiatives and persuading people to support them is a well-known challenge. However, scientific literature offers valuable insights. These include encouraging people to rely on rational thinking rather than emotions and providing information on how masks protect those around them. The fact that East Asian cultures tend to have a more positive relationship with the use of masks shows that, in principle, it is possible to make them acceptable. Data from studies suggest that, as we prepare for potential future pandemics, it may be time to move past polarization and reintroduce masks — not as a universal mandate but as an individual choice for many.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
COVID-19 Takes a Greater Toll on Kidneys Than Pneumonia
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This decline in kidney function, measured by the estimated glomerular filtration rate (eGFR), is particularly steep among individuals who require hospitalization for COVID-19.
METHODOLOGY:
- SARS-CoV-2, the virus that causes COVID-19, has been associated with acute kidney injury, but its potential impact on long-term kidney function remains unclear.
- Researchers investigated the decline in kidney function after COVID-19 vs pneumonia by including all hospitalized and nonhospitalized adults from the Stockholm Creatinine Measurements Project who had at least one eGFR measurement in the 2 years before a positive COVID-19 test result or pneumonia diagnosis.
- Overall, 134,565 individuals (median age, 51 years; 55.6% women) who had their first SARS-CoV-2 infection between February 2020 and January 2022 were included, of whom 13.3% required hospitalization within 28 days of their first positive COVID-19 test result.
- They were compared with 35,987 patients (median age, 71 years; 53.8% women) who were diagnosed with pneumonia between February 2018 and January 2020; 46.5% of them required hospitalization.
- The primary outcome measure focused on the mean annual change in eGFR slopes before and after each infection; the secondary outcome assessed was the annual change in postinfection eGFR slopes between COVID-19 and pneumonia cases.
TAKEAWAY:
- Before COVID-19, eGFR changes were minimal, but after the infection, the average decline increased to 4.1 (95% CI, 3.8-4.4) mL/min/1.73 m2; however, in the pneumonia cohort, a decline in eGFR was noted both before and after the infection.
- After COVID-19, the mean annual decline in eGFR was 3.4% (95% CI, 3.2%-3.5%), increasing to 5.4% (95% CI, 5.2%-5.6%) for those who were hospitalized.
- In contrast, the pneumonia group experienced an average annual decline of 2.3% (95% CI, 2.1%-2.5%) after the infection, which remained unchanged when analyzing only patients who were hospitalized.
- The risk for a 25% reduction in eGFR was higher in patients with COVID-19 than in those with pneumonia (hazard ratio [HR], 1.19; 95% CI, 1.07-1.34), with the risk being even higher among those who required hospitalization (HR, 1.42; 95% CI, 1.22-1.64).
IN PRACTICE:
“These findings help inform decisions regarding the need to monitor kidney function in survivors of COVID-19 and could have implications for policymakers regarding future healthcare planning and kidney service provision,” the authors wrote.
SOURCE:
This study was led by Viyaasan Mahalingasivam, MPhil, London School of Hygiene & Tropical Medicine, London, England. It was published online in JAMA Network Open.
LIMITATIONS:
This study lacked information on important confounders such as ethnicity and body mass index. The follow-up period was not long enough to fully evaluate the long-term association of COVID-19 with kidney function. Some individuals may have been misclassified as nonhospitalized if their first infection was mild and a subsequent infection required hospitalization.
DISCLOSURES:
This study was supported by grants from the National Institute for Health and Care Research, Njurfonden, Stig and Gunborg Westman Foundation, and the Swedish Research Council. One author reported receiving a Career Development Award from the National Institute for Health and Care Research, and another author reported receiving grants from Njurfonden, Stig and Gunborg Westman Foundation, Swedish Research Council, Swedish Heart Lung Foundation, and Region Stockholm during the conduct of the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Early Oseltamivir Benefits Hospitalized Influenza Patients
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
Most US Adults Plan to Skip Annual COVID Vaccines
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.