User login
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
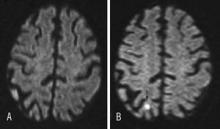
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.