User login
New SVS Task Force Explores Vascular Certification Program
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
VAM ’17 Will Be a ‘Spectacular Meeting’
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
SVS Now Accepting Abstracts for VAM 2017
Abstracts for the 2017 Vascular Annual Meeting are now being accepted. The submission site opened Monday, Nov. 14 for the meeting, to be held May 31 to June 3, 2017, in San Diego. Plenary sessions and exhibits will be June 1 to 3.
Participants may submit abstracts into any of 14 categories and a number of presentation types, including videos. In 2016, organizers selected approximately two-thirds of the submitted abstracts, and this year the VAM Program Committee is seeking additional venues for people to present their work in, including more sessions and other presentation formats.
Click here for abstract guidelines and more information. Abstracts themselves may be submitted here.
Abstracts for the 2017 Vascular Annual Meeting are now being accepted. The submission site opened Monday, Nov. 14 for the meeting, to be held May 31 to June 3, 2017, in San Diego. Plenary sessions and exhibits will be June 1 to 3.
Participants may submit abstracts into any of 14 categories and a number of presentation types, including videos. In 2016, organizers selected approximately two-thirds of the submitted abstracts, and this year the VAM Program Committee is seeking additional venues for people to present their work in, including more sessions and other presentation formats.
Click here for abstract guidelines and more information. Abstracts themselves may be submitted here.
Abstracts for the 2017 Vascular Annual Meeting are now being accepted. The submission site opened Monday, Nov. 14 for the meeting, to be held May 31 to June 3, 2017, in San Diego. Plenary sessions and exhibits will be June 1 to 3.
Participants may submit abstracts into any of 14 categories and a number of presentation types, including videos. In 2016, organizers selected approximately two-thirds of the submitted abstracts, and this year the VAM Program Committee is seeking additional venues for people to present their work in, including more sessions and other presentation formats.
Click here for abstract guidelines and more information. Abstracts themselves may be submitted here.
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
ARCADIA: Predicting risk of atrial cardiopathy poststroke
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.
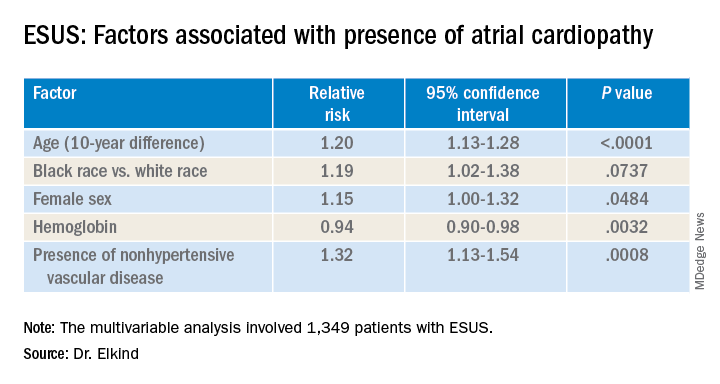
Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
REPORTING FROM ISC 2020
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE
Mobile stroke unit had clinical impact on EVT
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
FROM STROKE
Key clinical point: A mobile stroke unit (MSU) substantially reduced time to reperfusion therapies.
Major finding: Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations).
Study details: A prospective study of 100 stroke patients.
Disclosures: The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
Source: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.






