User login
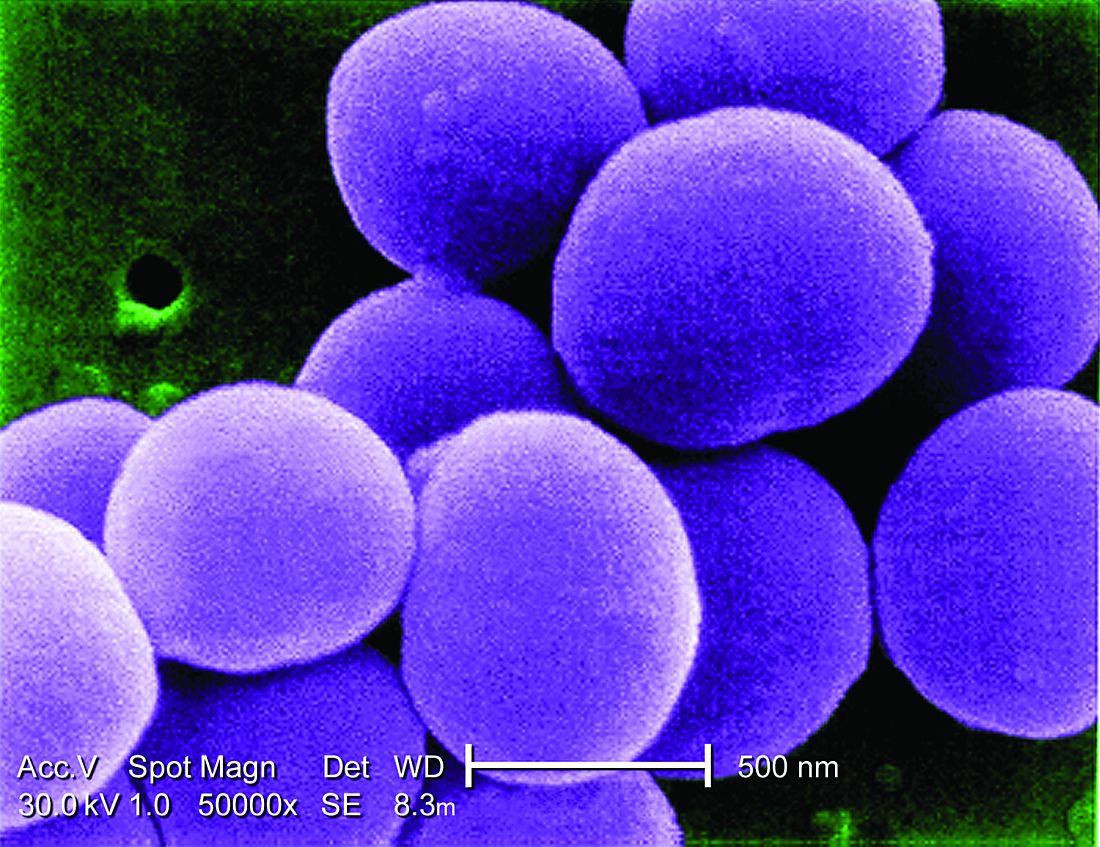
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.