User login
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
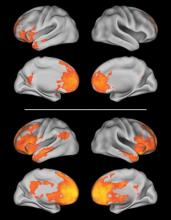
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.