User login
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
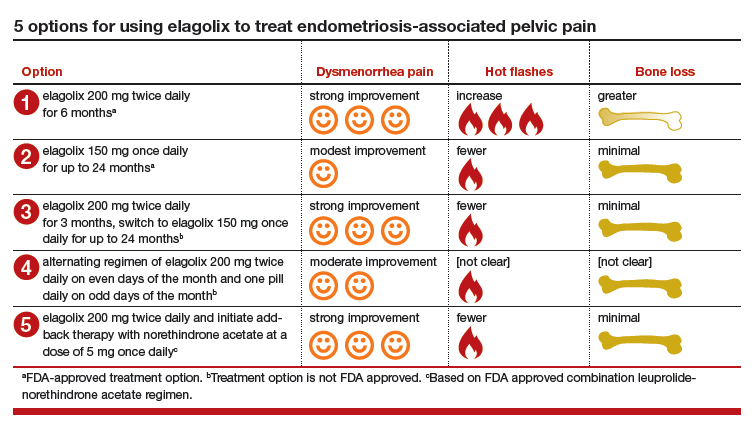
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3
Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.