User login
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
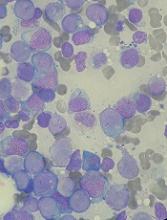
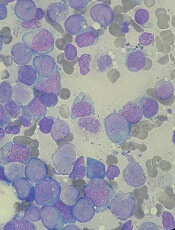
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()