User login
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
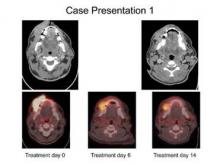
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
FROM A HEAD AND NECK CANCER SYMPOSIUM SPONSORED BY THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Of 10 evaluable head and neck cancer patients, 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter.
Data Source: In this small, ongoing pilot study smokers received 300 mg and nonsmokers 150 mg daily of neoadjuvant erlotinib.
Disclosures: Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.