User login
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.
Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
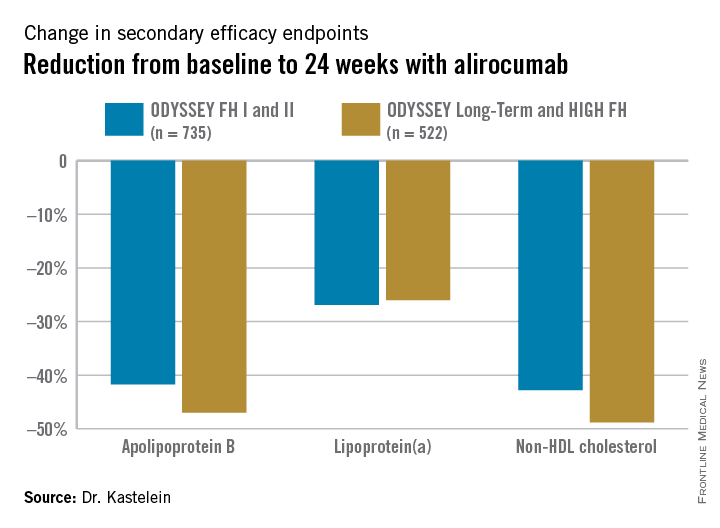
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.

Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
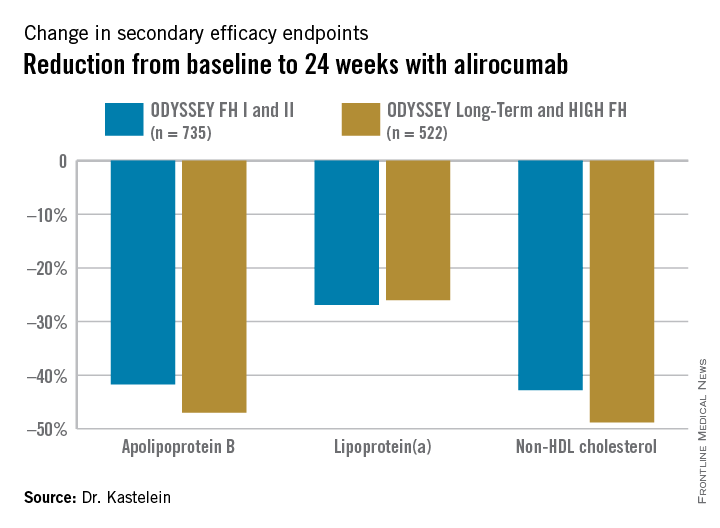
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.

Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
AT THE ESC CONGRESS 2015
Key clinical point: The PCSK9 inhibitor alirocumab lowers LDL cholesterol to unprecedented levels patients with heterozygous familial hypercholesterolemia.
Major finding: Of patients with heterozygous familial hypercholesterolemia with unacceptably high LDL levels despite intensive conventional lipid-lowering agents, 56% achieved an LDL cholesterol below 70 mg/dL after 24 weeks of alirocumab, compared with 1% of placebo-treated controls.
Data source: This analysis included 1,257 individuals with heterozygous familial hypercholesterolemia in four phase III, randomized, double-blind clinical trials who were randomized 2-to-1 to alirocumab or placebo plus background maximally tolerated conventional lipid-lowering medications and followed prospectively for 78 weeks.
Disclosures: The studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.