User login
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
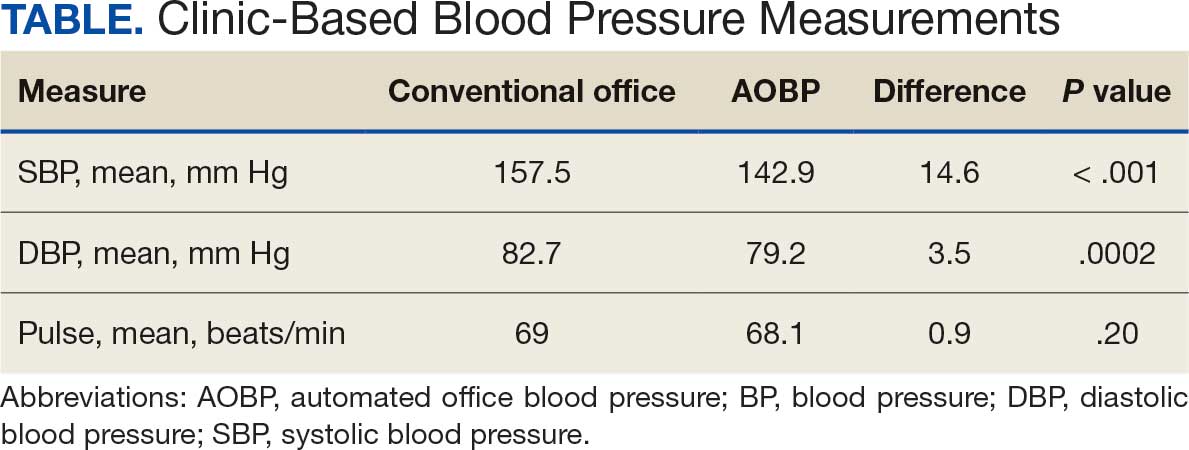
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).
A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
Hypertension remains one of the most important modifiable risk factors for the prevention of cardiovascular (CV) events. According to a population-based study, 25% of CV events (CV death, heart disease, coronary revascularization, stroke, or heart failure) are attributable to hypertension.1 Recent guidelines have emphasized the importance of accurate blood pressure (BP) measurement in facilitating appropriate hypertension diagnosis and management.2-4
Currently, there are different BP measurement methods endorsed by practice guidelines. These include conventional in-office measurement, 24-hour ambulatory BP monitoring (ABPM), home BP monitoring (HBPM), and automated office BP (AOBP) measurement.2-4 AOBP device protocols vary but generally involve devices automatically taking multiple BP measurements while the patient is unattended. These measurements are then presented as a single averaged reading, with individual BP values available for review by the clinician.
Researchers have found that AOBP measurements have a greater association with ABPM values and can mitigate the white coat effect observed in a substantial proportion of patients during in-clinic BP measurement.5 A meta-analysis found that the use of AOBP was associated with a 10.5 mm Hg reduction in systolic BP (SBP) compared with traditional office-based BP assessments.5 Similarly, a separate meta-analysis found that AOBP SBP measures were on average 14.5 mm Hg lower than routine office or research setting values.6 In addition, CV risk outcomes data support the use of AOBP to screen and manage patients with hypertension. The Cardiovascular Health Awareness Program (CHAP) study used AOBP values to determine the risk for CV events (myocardial infarction, congestive heart failure, and stroke) in community-based patients aged ≥ 65 years.7 The study showed a significantly higher risk of CV events in patients with an SBP of 135 to 144 mm Hg and a diastolic BP (DBP) of 80 to 89 mm Hg. Therefore, the CHAP study researchers suggested an AOBP target of < 135/85 mm Hg to decrease the risk of CV events.7The landmark SPRINT trial, which was a major contributor to the development of BP target recommendations in guidelines, utilized AOBP to classify hypertension and guide management.2-4,8 SPRINT ultimately showed that intensive BP-lowering treatment (to SBP < 120 mm Hg) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause mortality.8 Other evaluations found a close association between AOBP values and left ventricular mass index and carotid artery wall thickness as surrogate markers for end-organ damage.9,10 These data show AOBP as a reliable method to guide antihypertensive therapy interventions in the clinical setting.
Considering these proposed advantages, the 2017 Canadian guidelines for hypertension management recommend AOBP as the preferred method for clinic-based BP measurement, and the 2018 European Society of Cardiology/European Society of Hypertension blood pressure guidelines recommend the use of AOBP when feasible.3,4 The 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults also discusses AOBP as a method to minimize potential confounders in BP values.2
This study evaluated the difference between AOBP and conventional in-office BP measurements obtained during cardiology clinic visits at the West Palm Beach Veterans Affairs Medical Center (WPBVAMC).
METHODS
A retrospective review of AOBP measurements was performed at the WPBVAMC cardiology clinic between May 26, 2017, and February 19, 2019. These AOBP measurements were taken at the discretion of a nurse or other clinician after initial, conventional BP measurements had been taken as part of clinic check-in procedures. No formal protocols dictated the use or timing of AOBP measurements. Similarly, the AOBP results were factored into clinical care decisions.
Clinicians at the cardiology clinic used AOBP averages that were derived using the BpTRU BPM-100 (BpTRU Medical Devices) meter, which averaged 5 BP readings taken at 1-minute intervals. Clinicians selected cuff size based on manufacturer recommendations. The testing was done with the patient seated alone in either a nursing triage area or a clinic office.
Data collected during the retrospective review included the clinician associated with the visit, the patient’s physical location and accompaniment status during AOBP measurement, conventionally measured BP and heart rates, and AOBP-derived BP and heart rate averages. Differences in BP values were compared with the paired t test, while binary comparisons were conducted through the McNemar test. Data collection and analysis were performed using Microsoft Excel.
During data collection, all information was stored in a secure drive accessible only to the investigators. The project was approved by the West Palm Beach Veterans Affairs Healthcare System Research and Development Committee as a nonresearch activity in accordance with Veterans Health Administration Handbook 1058.05; thus, institutional review board approval was not required.
RESULTS
Ninety-five nonconsecutive patients were included in the analysis. AOBP measurements were taken with the patient sitting alone in either a clinic office (n = 83) or nursing triage area (n = 12). Most patients were coming in for follow-up appointments; 13 patients (14%) had appointments related to a 24-hour ABPM session.
The mean SBP and DBP values were lower for the AOBP measurements vs the conventional BP measurements (mean SBP difference, 14.6 mm Hg; P < .001; mean DBP difference, 3.5 mm Hg; P = .0002) (Table). There were no appreciable differences in heart rates. The white coat effect was suggested based on an SBP reduction of > 20 mm Hg from conventional to AOBP measurements in 22 patients (23%), a DBP reduction of > 10 mm Hg in 21 patients (22%), and a reduction in both values in 8 patients (8%).

A controlled BP (< 130/80 mm Hg) was more common in the AOBP group than in the conventional group (22% vs 7%, respectively; P =.001).2 Review of conventional BP measurements indicated that 11 patients had systolic readings ≥ 180 mm Hg, 2 had diastolic readings ≥ 110 mm Hg, and 1 had a reading that was ≥ 180/110 mm Hg. AOBP measurements indicated that these 14 patients had SBP readings < 180 mm Hg and DBP readings < 110 mm Hg. The use of AOBP measurements may have mitigated unnecessary emergency room visits for these patients.
On review of clinic notes and actions associated with episodes of AOBP testing during routine follow-up clinic appointments, AOBP was determined to be useful with regard to clinical decision-making for 65 (79%) patients. Impacts of AOBP inclusion vs conventional BP assessments included clinician notation of AOBP, support for making changes that would have been considered based on conventional BP assessment. AOBP results gave support to forgoing a therapeutic intervention (ie, therapy addition or intensification) that may have been pursued based on conventional BP measurements in 25 of 82 patients (30%). These data suggest that AOBP readings can be useful and actionable by clinicians.
DISCUSSION
The findings of this study add to the growing evidence regarding AOBP use, application, and advantages in clinical practice. In this evaluation, the mean difference in SBP and DBP was 14.6 mm Hg and 3.5 mm Hg, respectively, from the conventional office measurements to the AOBP measurements. This difference is similar to that reported by the CAMBO trial and other evaluations, where the use of AOBP measurements corresponded to a reduction in SBP of between 10 and 20 mm Hg vs conventional measures.5,11-18
These findings showed a significantly higher percentage of controlled BP values (< 130/80 mm Hg) with AOBP values compared with conventional office measurements. The data supported the decision to defer antihypertensive therapy intervention in 30% of patients. Without AOBP data, patients may have been classified as uncontrolled, prompting therapy addition or intensification that could increase the risk of adverse events. Additionally, 14 patients would have met the criteria for hypertensive urgency under the guidelines at that time.2 With the use of AOBP readings, none of these patients were identified as having a hypertensive urgency, and they avoided an acute care referral or urgent intervention.
The discrepancy between AOBP and conventional office BP measurements suggested a white coat effect based on SBP and DBP readings in 22 (23%) and 21 (22%) patients, respectively. Practice guidelines recommend ABPM to mitigate a potential white coat effect.2-4 However, ABPM can be inconvenient for patients, as they need to travel to and from the clinic for fitting and removal (assuming that a facility has the device available for patient use). In addition, some patients may find it uncomfortable. Based on the correlation between AOBP and awake ABPM values, AOBP represents a feasible way to identify a white coat effect.
AOBP monitoring does not appear to be affected by the type of practice setting, as it has been evaluated in a variety of locations, including community-based pharmacies, primary care offices, and waiting rooms.12,19-22 However, potential AOBP implementation challenges may include office space constraints, clinician perception that it will delay workflow, and device cost. Costs associated with an AOBP meter vary widely based on device and procurement source, but have been estimated to range from $650 to > $2000.23 Published reports have described how to overcome AOBP implementation barriers.24,25
Limitations
The results of this evaluation should be interpreted cautiously due to several limitations. First, the retrospective study was conducted at a single clinic that may not be representative of other Veterans Health Administration or community-based populations. In addition, patient data such as age, sex, and body mass index were not available. AOBP measurements were obtained at the discretion of the clinician and not according to a prespecified protocol.
Conclusions
This analysis showed AOBP measurement leads to a greater percentage of controlled BP values compared with conventional office BP measurement, positioning it as a way to reduce BP misclassification, prevent potentially unnecessary therapeutic interventions, and mitigate the white coat effect.
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
- Cheng S, Claggett B, Correia AW, et al. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820-828. doi.org/10.1161/CIRCULATIONAHA.113.008506
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi:10.1161/HYP.0000000000000066
- Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. doi:10.1016/j.cjca.2017.03.005
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73(2):481-490. doi:10.1161/HYPERTENSIONAHA.118.12079
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension - a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351-362. doi:10.1001/jamainternmed.2018.6551
- Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med. 2008;46(6):537-544. doi:10.1016/j.ypmed.2008.02.005
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Andreadis EA, Agaliotis GD, Angelopoulos ET, et al. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661-666. doi:10.1038/ajh.2011.38
- Campbell NRC, McKay DW, Conradson H, et al. Automated oscillometric blood pressure versus auscultatory blood pressure as a predictor of carotid intima-medial thickness in male firefighters. J Hum Hypertens. 2007;21(7):588-590. doi:10.1038/sj.jhh.1002190
- Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi:10.1136/bmj.d286
- Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi:10.1186/1471-2261-5-18
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280-286. doi:10.1097/HJH.0b013e32831b9e6b
- Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14(3):108-111. doi:10.1097/MBP.0b013e32832c5167
- Myers MG, Valdivieso M, Kiss A. Optimum frequency of office blood pressure measurement using an automated sphygmomanometer. Blood Press Monit. 2008;13(6):333-338. doi:10.1097/MBP.0b013e3283104247
- Myers MG. A proposed algorithm for diagnosing hypertension using automated office blood pressure measurement. J Hypertens. 2010;28(4):703-708. doi:10.1097/HJH.0b013e328335d091
- Godwin M, Birtwhistle R, Delva D, et al. Manual and automated office measurements in relation to awake ambulatory blood pressure monitoring. Fam Pract. 2011;28(1):110-117. doi:10.1093/fampra/cmq067
- Myers MG, Valdivieso M, Chessman M, Kiss A. Can sphygmomanometers designed for self-measurement of blood pressure in the home be used in office practice? Blood Press Monit. 2010;15(6):300-304. doi:10.1097/MBP.0b013e328340d128
- Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. doi:10.1016/j.cjca.2016.02.066
- Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721-724. doi:10.1111/jch.12820
- Myers MG, Kaczorowski J, Dawes M, Godwin M. Automated office blood pressure measurement in primary care. Can Fam Physician. 2014;60(2):127-132.
- Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204-208. doi:10.1097/MBP.0000000000000133
- Yarows SA. What is the Cost of Measuring a Blood Pressure? Ann Clin Hypertens. 2018;2:59-66. doi:10.29328/journal.ach.1001012
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10.1001/jama.282.15.1458
- Doane J, Buu J, Penrod MJ, et al. Measuring and managing blood pressure in a primary care setting: a pragmatic implementation study. J Am Board Fam Med. 2018;31(3):375-388. doi:10.3122/jabfm.2018.03.170450
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Assessment of Automated vs Conventional Blood Pressure Measurements in a Veterans Affairs Clinical Practice Setting
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Three Anomalies and a Complication: Ruptured Noncoronary Sinus of Valsalva Aneurysm, Atrial Septal Aneurysm, and Patent Foramen Ovale
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
Veterans Face Long Delays in ATTR-CM Diagnosis After HF
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Veterans received a diagnosis of transthyretin amyloid cardiomyopathy (ATTR-CM) a median of 490 days after heart failure (HF) diagnosis. Those who had atrial fibrillation, coronary artery disease, or chronic kidney disease experienced longer diagnostic delays, whereas older or Black patients experienced shorter delays.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Veterans Health Administration to quantify the time from HF diagnosis to ATTR-CM diagnosis and identify predictors of delays in diagnosis.
- They included veterans with HF and incident ATTR-CM diagnosed between 2016 and 2022; these veterans were identified using an algorithm based on diagnoses and medications.
- The final analysis included 2557 veterans, with a median age of 80.5 years; 99.5% were men, and 56.3% were White individuals.
- Assessments captured the time from the first HF diagnosis, the first outpatient prescription for a loop diuretic, and the first hospitalization for HF to the first ATTR-CM diagnosis, along with demographics and comorbidities.
- The primary outcome was the number of days from incident HF diagnosis to incident ATTR-CM diagnosis; a delay of > 6 months was considered meaningful.
TAKEAWAY:
- The median time from HF diagnosis to ATTR-CM diagnosis was 490 days. Overall, 65% of veterans experienced diagnostic delays > 6 months, and > 25% had delays longer than 3 years.
- Factors associated with a shorter time to ATTR-CM diagnosis included Black race (adjusted odds ratio [aOR], 0.71; 95% CI, 0.57-0.88) and older age (aOR per 10 years, 0.66; 95% CI, 0.59-0.73).
- The likelihood of longer diagnostic delays was higher in patients with coronary artery disease (aOR, 1.38; 95% CI, 1.15-1.64) and chronic kidney disease (aOR, 1.79; 95% CI, 1.50-2.15). Those with atrial fibrillation were more likely to have longer delays, although the lower bound of 95% CI was borderline (aOR, 1.21; 95% CI, 1.00-1.45).
- Among veterans with prior prescriptions of loop diuretics, the median time from the first prescription to ATTR-CM diagnosis was 835 days. Among those with a prior hospitalization for HF, the median time from hospitalization to ATTR-CM diagnosis was 300 days.
IN PRACTICE:
“This study supports the need for increased testing for ATTR-CM, thorough evaluation of cardiomyopathy etiologies at the time of HF diagnosis, and the need for new interventions that shorten the diagnostic delay in ATTR-CM,” the researchers reported.
SOURCE:
This study was led by Gabriela Spencer-Bonilla, MD, MSc, of Stanford University School of Medicine in Stanford, California. It was published online on JACC .
LIMITATIONS:
The analysis was limited to veterans engaged long enough for diagnoses of both HF and ATTR-CM. The findings may not be generalized to women given the predominantly male cohort. Echocardiography and ATTR subtype or genetic data were unavailable.
DISCLOSURES:
This study received funding from AstraZeneca. Four authors reported being the employees and stockholders of AstraZeneca. Several authors reported receiving research funding, consulting fees, and/or having equity in multiple pharmaceutical and healthcare companies, including Novo Nordisk, Bridge Bio, and Pfizer. Two authors reported receiving support from the American Heart Association, with one of them also receiving support from the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Following the Hyperkalemia Trail: A Case Report of ECG Changes and Treatment Responses
Following the Hyperkalemia Trail: A Case Report of ECG Changes and Treatment Responses
Hyperkalemia involves elevated serum potassium levels (> 5.0 mEq/L) and represents an important electrolyte disturbance due to its potentially severe consequences, including cardiac effects that can lead to dysrhythmia and even asystole and death.1,2 In a US Medicare population, the prevalence of hyperkalemia has been estimated at 2.7% and is associated with substantial health care costs.3 The prevalence is even more marked in patients with preexisting conditions such as chronic kidney disease (CKD) and heart failure.4,5
Hyperkalemia can result from multiple factors, including impaired renal function, adrenal disease, adverse drug reactions of angiotensin-converting enzyme inhibitors (ACEIs) and other medications, and heritable mutations.6 Hyperkalemia poses a considerable clinical risk, associated with adverse outcomes such as myocardial infarction and increased mortality in patients with CKD.5,7,8 Electrocardiographic (ECG) changes associated with hyperkalemia play a vital role in guiding clinical decisions and treatment strategies.9 Understanding the pathophysiology, risk factors, and consequences of hyperkalemia, as well as the significance of ECG changes in its management, is essential for health care practitioners.
Case Presentation
An 81-year-old Hispanic man with a history of hypertension, hypothyroidism, gout, and CKD stage 3B presented to the emergency department with progressive weakness resulting in falls and culminating in an inability to ambulate independently. Additional symptoms included nausea, diarrhea, and myalgia. His vital signs were notable for a pulse of 41 beats/min. The physical examination was remarkable for significant weakness of the bilateral upper extremities, inability to bear his own weight, and bilateral lower extremity edema. His initial ECG upon arrival showed bradycardia with wide QRS, absent P waves, and peaked T waves (Figure 1a). These findings differed from his baseline ECG taken 1 year earlier, which showed sinus rhythm with premature atrial complexes and an old right bundle branch block (Figure 1b).
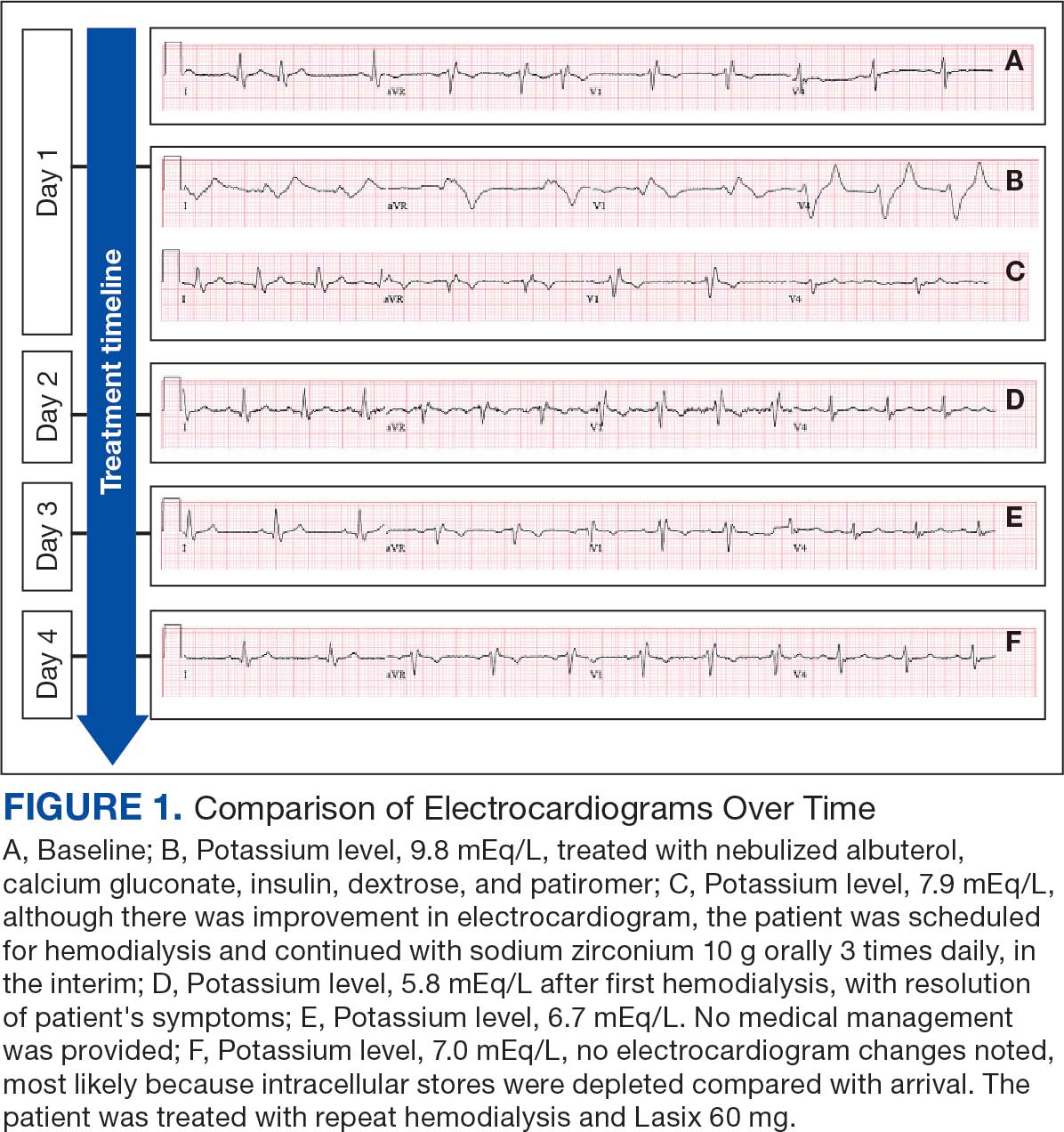
Medication review revealed that the patient was currently prescribed 100 mg allopurinol daily, 2.5 mg amlodipine daily, 10 mg atorvastatin at bedtime, 4 mg doxazosin daily, 112 mcg levothyroxine daily, 100 mg losartan daily, 25 mg metoprolol daily, and 0.4 mg tamsulosin daily. The patient had also been taking over-the-counter indomethacin for knee pain.
Based on the ECG results, he was treated with 0.083%/6 mL nebulized albuterol, 4.65 Mq/250 mL saline solution intravenous (IV) calcium gluconate, 10 units IV insulin with concomitant 50%/25 mL IV dextrose and 8.4 g of oral patiromer suspension. IV furosemide was held due to concern for renal function. The decision to proceed with hemodialysis was made. Repeat laboratory tests were performed, and an ECG obtained after treatment initiation but prior to hemodialysis demonstrated improvement of rate and T wave shortening (Figure 1c). The serum potassium level dropped from 9.8 mEq/L to 7.9 mEq/L (reference range, 3.5-5.0 mEq/L) (Table 1).
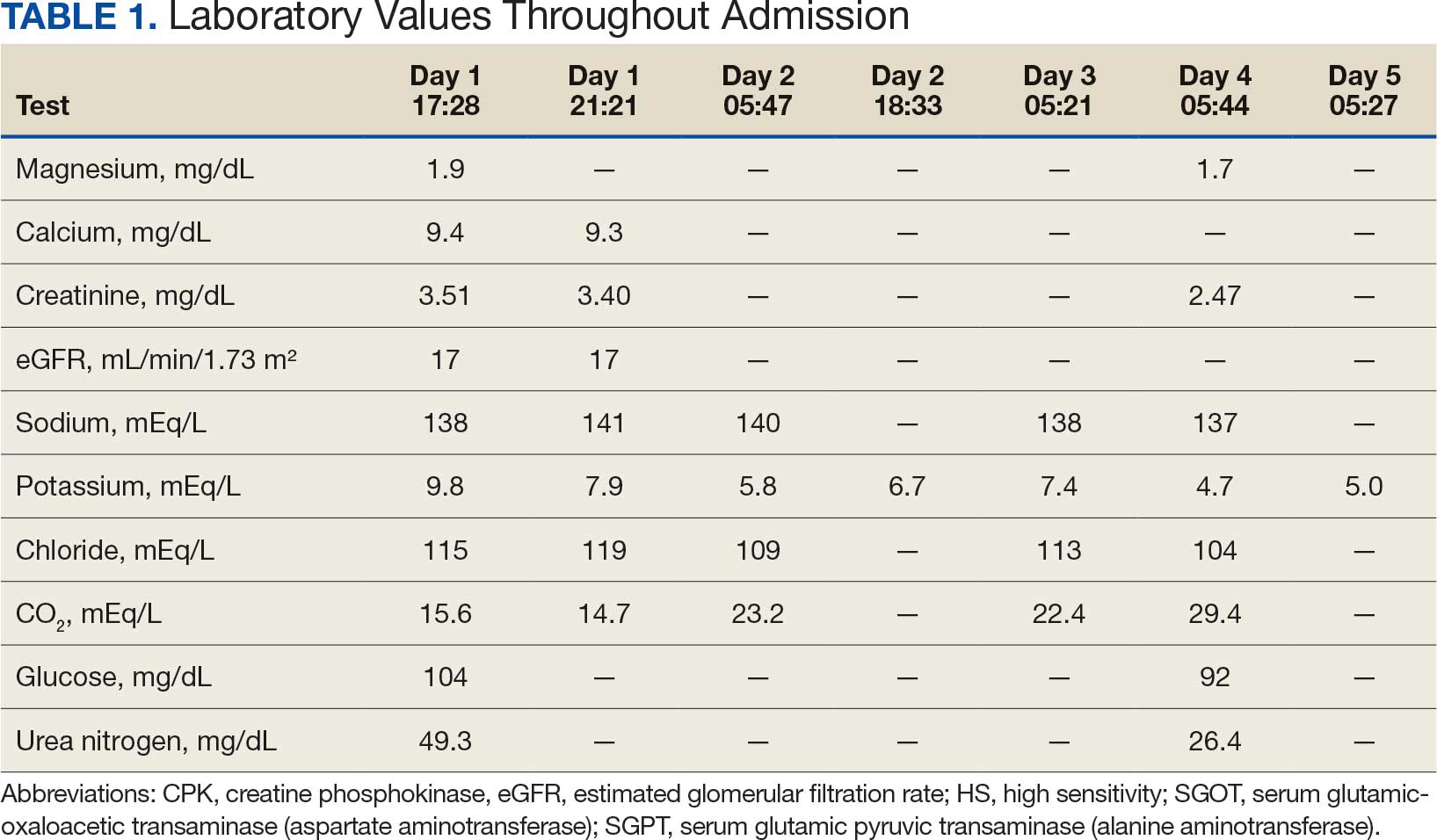
In addition to hemodialysis, sodium zirconium 10 g orally 3 times daily was added. Laboratory test results and an ECG was performed after dialysis continued to demonstrate improvement (Figure 1d). The patient’s potassium level decreased to 5.8 mEq/L, with the ECG demonstrating stability of heart rate and further improvement of the PR interval, QRS complex, and T waves.
Despite the established treatment regimen, potassium levels again rose to 6.7 mEq/L, but there were no significant changes in the ECG, and thus no medication changes were made (Figure 1e). Subsequent monitoring demonstrated a further increase in potassium to 7.4 mEq/L, with an ECG demonstrating a return to the baseline of 1 year prior. The patient underwent hemodialysis again and was given oral furosemide 60 mg every 12 hours. The potassium concentration after dialysis decreased to 4.7 mEq/L and remained stable, not going above 5.0 mEq/L on subsequent monitoring. The patient had resolution of all symptoms and was discharged.
Discussion
We have described in detail the presentation of each pathology and mechanisms of each treatment, starting with the patient’s initial condition that brought him to the emergency room—muscle weakness. Skeletal muscle weakness is a common manifestation of hyperkalemia, occurring in 20% to 40% of cases, and is more prevalent in severe elevations of potassium. Rarely, the weakness can progress to flaccid paralysis of the patient’s extremities and, in extreme cases, the diaphragm.
Muscle weakness progression occurs in a manner that resembles Guillain-Barré syndrome, starting in the lower extremities and ascending toward the upper extremities.10 This is known as secondary hyperkalemic periodic paralysis. Hyperkalemia lowers the transmembrane gradient in neurons, leading to neuronal depolarization independent of the degree of hyperkalemia. If the degree of hyperkalemia is large enough, this depolarization inactivates voltage-gated sodium channels, making neurons refractory to excitation. Electromyographical studies have shown reduction in the compounded muscle action potential.11 The transient nature of this paralysis is reflected by rapid correction of weakness and paralysis when the electrolyte disorder is corrected.
The patient in this case also presented with bradycardia. The ECG manifestations of hyperkalemia can include atrial asystole, intraventricular conduction disturbances, peaked T waves, and widened QRS complexes. However, some patients with renal insufficiency may not exhibit ECG changes despite significantly elevated serum potassium levels.12
The severity of hyperkalemia is crucial in determining the associated ECG changes, with levels > 6.0 mEq/L presenting with abnormalities.13 ECG findings alone may not always accurately reflect the severity of hyperkalemia, as up to 60% of patients with potassium levels > 6.0 mEq/L may not show ECG changes.14 Additionally, extreme hyperkalemia can lead to inconsistent ECG findings, making it challenging to rely solely on ECG for diagnosis and monitoring.8 The level of potassium that causes these effects varies widely through patient populations.
The main mechanism by which hyperkalemia affects the heart’s conduction system is through voltage differences across the conduction fibers and eventual steady-state inactivation of sodium channels. This combination of mechanisms shortens the action potential duration, allowing more cardiomyocytes to undergo synchronized depolarization. This amalgamation of cardiomyocytes repolarizing can be reflected on ECGs as peaked T waves. As the action potential decreases, there is a period during which cardiomyocytes are prone to tachyarrhythmias and ventricular fibrillation.
A reduced action potential may lead to increased rates of depolarization and thus conduction, which in some scenarios may increase heart rate. As the levels of potassium rise, intracellular accumulation impedes the entry of sodium by decreasing the cation gradient across the cell membrane. This effectively slows the sinus nodes and prolongs the QRS by slowing the overall propagation of action potentials. By this mechanism, conduction delays, blocks, or asystole are manifested. The patient in this case showed conduction delays, peaked T waves, and disappearance of P waves when he first arrived.
Hyperkalemia Treatment
Hyperkalemia develops most commonly due to acute or chronic kidney diseases, as was the case with this patient. The patient’s hyperkalemia was also augmented by the use of nonsteroidal anti-inflammatory drugs (NSAIDs), which can directly affect renal function. A properly functioning kidney is responsible for excretion of up to 90% of ingested potassium, while the remainder is excreted through the gastrointestinal (GI) tract. Definitive treatment of hyperkalemia is mitigated primarily through these 2 organ systems. The treatment also includes transitory mechanisms of potassium reduction. The goal of each method is to preserve the action potential of cardiomyocytes and myocytes. This patient presented with acute symptomatic hyperkalemia and received various medications to acutely, transitorily, and definitively treat it.
Initial therapy included calcium gluconate, which functions to stabilize the myocardial cell membrane. Hyperkalemia decreases the resting membrane action potential of excitable cells and predisposes them to early depolarization and thus dysrhythmias. Calcium decreases the threshold potential across cells and offsets the overall gradient back to near normal levels.15 Calcium can be delivered through calcium gluconate or calcium chloride. Calcium chloride is not preferred because extravasation can cause pain, blistering and tissue ischemia. Central venous access is required, potentially delaying prompt treatment. Calcium acts rapidly after administration—within 1 to 3 minutes—but only lasts 30 to 60 minutes.16 Administration of calcium gluconate can be repeated as often as necessary, but patients must be monitored for adverse effects of calcium such as nausea, abdominal pain, polydipsia, polyuria, muscle weakness, and paresthesia. Care must be taken when patients are taking digoxin, because calcium may potentiate toxicity.17 Although calcium provides immediate benefits it does little to correct the underlying cause; other medications are required to remove potassium from the body.
Two medication classes have been proven to shift potassium intracellularly. The first are β-2 agonists, such as albuterol/levalbuterol, and the second is insulin. Both work through sodium-potassium-ATPase in a direct manner. β-2 agonists stimulate sodium-potassium-ATPase to move more potassium intracellularly, but these effects have been seen only with high doses of albuterol, typically 4× the standard dose of 0.5 mg in nebulized solutions to achieve decreases in potassium of 0.3 to 0.6 mEq/L, although some trials have reported decreases of 0.62 to 0.98 mEq/L.15,18 These potassium-lowering effects of β-2 agonist are modest, but can be seen 20 to 30 minutes after administration and persist up to 1 to 2 hours. β-2 agonists are also readily affected by β blockers, which may reduce or negate the desired effect in hyperkalemia. For these reasons, a β-2 agonist should not be given as monotherapy and should be provided as an adjuvant to more independent therapies such as insulin. Insulin binds to receptors on muscle cells and increases the quantity of sodium-potassium-ATPase and glucose transporters. With this increase in influx pumps, surrounding tissues with higher resting membrane potentials can absorb the potassium load, thereby protecting cardiomyocytes.
Potassium Removal
Three methods are currently available to remove potassium from the body: GI excretion, renal excretion, and direct removal from the bloodstream. Under normal physiologic conditions, the kidneys account for about 90% of the body’s ability to remove potassium. Loop diuretics facilitate the removal of potassium by increasing urine production and have an additional potassium-wasting effect. Although the onset of action of loop diuretics is typically 30 to 60 minutes after oral administration, their effect can last for several hours. In this patient, furosemide was introduced later in the treatment plan to manage recurring hyperkalemia by enhancing renal potassium excretion.
Potassium binders such as patiromer act in the GI tract, effectively reducing serum potassium levels although with a slower onset of action than furosemide, generally taking hours to days to exert its effect. Both medications illustrate a tailored approach to managing potassium levels, adapted to the evolving needs and renal function of the patient. The last method is using hemodialysis—by far the most rapid method to remove potassium, but also the most invasive. The different methods of treating hyperkalemia are summarized in Table 2. This patient required multiple days of hemodialysis to completely correct the electrolyte disorder. Upon discharge, the patient continued oral furosemide 40 mg daily and eventually discontinued hemodialysis due to stable renal function.
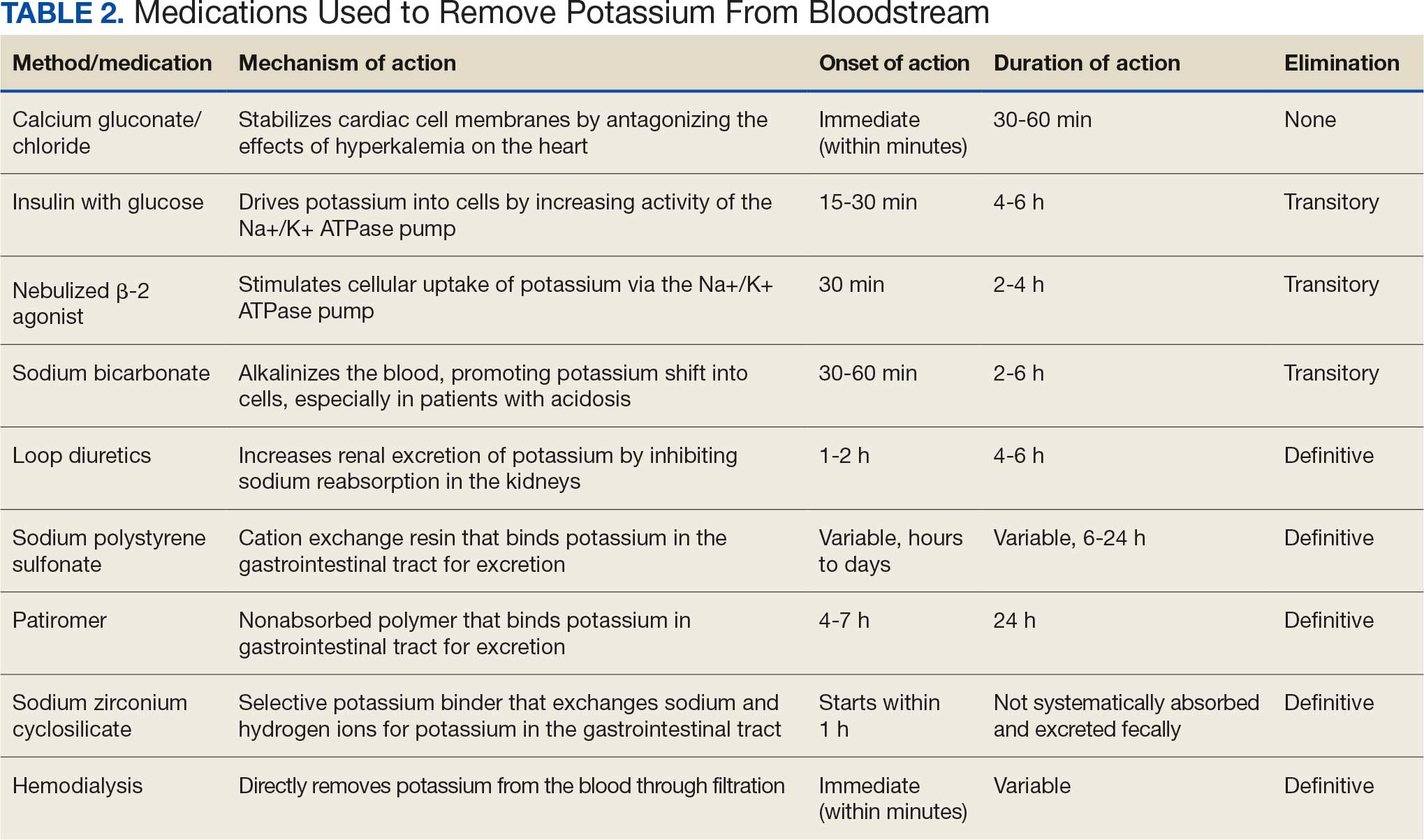
Often, after correcting an inciting event, potassium stores in the body eventually stabilize and do not require additional follow-up. Patients prone to hyperkalemia should be thoroughly educated on medications to avoid (NSAIDs, ACEIs/ARBs, trimethoprim), an adequate low potassium diet, and symptoms that may warrant medical attention.19
Conclusions
This case illustrates the importance of recognizing the spectrum of manifestations of hyperkalemia, which ranged from muscle weakness to cardiac dysrhythmias. Management strategies for the patient included stabilization of cardiac membranes, potassium shifting, and potassium removal, each tailored to the patient’s individual clinical findings.
The case further illustrates the critical role of continuous monitoring and dynamic adjustment of therapeutic strategies in response to evolving clinical and laboratory findings. The initial and subsequent ECGs, alongside laboratory tests, were instrumental in guiding the adjustments needed in the treatment regimen, ensuring both the efficacy and safety of the interventions. This proactive approach can mitigate the risk of recurrent hyperkalemia and its complications.
- Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381-401. doi:10.1146/annurev.physiol.010908.163241 2.
- Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. In: StatPearls. StatPearls Publishing; September 4, 2023. Accessed October 22, 2025.
- Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36:1333-1341. doi:10.1080/03007995.2020.1775072
- Loutradis C, Tolika P, Skodra A, et al. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested case-control study. Am J Nephrol. 2015;42:351-360. doi:10.1159/000442393
- Grodzinsky A, Goyal A, Gosch K, et al. Prevalence and prognosis of hyperkalemia in patients with acute myocardial infarction. Am J Med. 2016;129:858-865. doi:10.1016/j.amjmed.2016.03.008
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(suppl 3):iii2-iii11. doi:10.1093/ndt/gfz206
- Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90-100. doi:10.2215/CJN.01730215
- Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28:3155-3165. doi:10.1681/ASN.2016121344
- Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721-729. doi:10.1053/ajem.2000.7344
- Kimmons LA, Usery JB. Acute ascending muscle weakness secondary to medication-induced hyperkalemia. Case Rep Med. 2014;2014:789529. doi:10.1155/2014/789529
- Naik KR, Saroja AO, Khanpet MS. Reversible electrophysiological abnormalities in acute secondary hyperkalemic paralysis. Ann Indian Acad Neurol. 2012;15:339-343. doi:10.4103/0972-2327.104354
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324-330. doi:10.2215/CJN.04611007
- Larivée NL, Michaud JB, More KM, Wilson JA, Tennankore KK. Hyperkalemia: prevalence, predictors and emerging treatments. Cardiol Ther. 2023;12:35-63. doi:10.1007/s40119-022-00289-z
- Shingarev R, Allon M. A physiologic-based approach to the treatment of acute hyperkalemia. Am J Kidney Dis. 2010;56:578-584. doi:10.1053/j.ajkd.2010.03.014
- Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40-47.
- Ng KE, Lee CS. Updated treatment options in the management of hyperkalemia. U.S. Pharmacist. February 16, 2017. Accessed October 1, 2025. www.uspharmacist.com/article/updated-treatment-options-in-the-management-of-hyperkalemia
- Quick G, Bastani B. Prolonged asystolic hyperkalemic cardiac arrest with no neurologic sequelae. Ann Emerg Med. 1994;24:305-311. doi:10.1016/s0196-0644(94)70144-x 18.
- Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med. 1989;110:426-429. doi:10.7326/0003-4819-110-6-42619.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4 suppl):S117-S314. doi:10.1016/j.kint.2023.10.018
Hyperkalemia involves elevated serum potassium levels (> 5.0 mEq/L) and represents an important electrolyte disturbance due to its potentially severe consequences, including cardiac effects that can lead to dysrhythmia and even asystole and death.1,2 In a US Medicare population, the prevalence of hyperkalemia has been estimated at 2.7% and is associated with substantial health care costs.3 The prevalence is even more marked in patients with preexisting conditions such as chronic kidney disease (CKD) and heart failure.4,5
Hyperkalemia can result from multiple factors, including impaired renal function, adrenal disease, adverse drug reactions of angiotensin-converting enzyme inhibitors (ACEIs) and other medications, and heritable mutations.6 Hyperkalemia poses a considerable clinical risk, associated with adverse outcomes such as myocardial infarction and increased mortality in patients with CKD.5,7,8 Electrocardiographic (ECG) changes associated with hyperkalemia play a vital role in guiding clinical decisions and treatment strategies.9 Understanding the pathophysiology, risk factors, and consequences of hyperkalemia, as well as the significance of ECG changes in its management, is essential for health care practitioners.
Case Presentation
An 81-year-old Hispanic man with a history of hypertension, hypothyroidism, gout, and CKD stage 3B presented to the emergency department with progressive weakness resulting in falls and culminating in an inability to ambulate independently. Additional symptoms included nausea, diarrhea, and myalgia. His vital signs were notable for a pulse of 41 beats/min. The physical examination was remarkable for significant weakness of the bilateral upper extremities, inability to bear his own weight, and bilateral lower extremity edema. His initial ECG upon arrival showed bradycardia with wide QRS, absent P waves, and peaked T waves (Figure 1a). These findings differed from his baseline ECG taken 1 year earlier, which showed sinus rhythm with premature atrial complexes and an old right bundle branch block (Figure 1b).

Medication review revealed that the patient was currently prescribed 100 mg allopurinol daily, 2.5 mg amlodipine daily, 10 mg atorvastatin at bedtime, 4 mg doxazosin daily, 112 mcg levothyroxine daily, 100 mg losartan daily, 25 mg metoprolol daily, and 0.4 mg tamsulosin daily. The patient had also been taking over-the-counter indomethacin for knee pain.
Based on the ECG results, he was treated with 0.083%/6 mL nebulized albuterol, 4.65 Mq/250 mL saline solution intravenous (IV) calcium gluconate, 10 units IV insulin with concomitant 50%/25 mL IV dextrose and 8.4 g of oral patiromer suspension. IV furosemide was held due to concern for renal function. The decision to proceed with hemodialysis was made. Repeat laboratory tests were performed, and an ECG obtained after treatment initiation but prior to hemodialysis demonstrated improvement of rate and T wave shortening (Figure 1c). The serum potassium level dropped from 9.8 mEq/L to 7.9 mEq/L (reference range, 3.5-5.0 mEq/L) (Table 1).

In addition to hemodialysis, sodium zirconium 10 g orally 3 times daily was added. Laboratory test results and an ECG was performed after dialysis continued to demonstrate improvement (Figure 1d). The patient’s potassium level decreased to 5.8 mEq/L, with the ECG demonstrating stability of heart rate and further improvement of the PR interval, QRS complex, and T waves.
Despite the established treatment regimen, potassium levels again rose to 6.7 mEq/L, but there were no significant changes in the ECG, and thus no medication changes were made (Figure 1e). Subsequent monitoring demonstrated a further increase in potassium to 7.4 mEq/L, with an ECG demonstrating a return to the baseline of 1 year prior. The patient underwent hemodialysis again and was given oral furosemide 60 mg every 12 hours. The potassium concentration after dialysis decreased to 4.7 mEq/L and remained stable, not going above 5.0 mEq/L on subsequent monitoring. The patient had resolution of all symptoms and was discharged.
Discussion
We have described in detail the presentation of each pathology and mechanisms of each treatment, starting with the patient’s initial condition that brought him to the emergency room—muscle weakness. Skeletal muscle weakness is a common manifestation of hyperkalemia, occurring in 20% to 40% of cases, and is more prevalent in severe elevations of potassium. Rarely, the weakness can progress to flaccid paralysis of the patient’s extremities and, in extreme cases, the diaphragm.
Muscle weakness progression occurs in a manner that resembles Guillain-Barré syndrome, starting in the lower extremities and ascending toward the upper extremities.10 This is known as secondary hyperkalemic periodic paralysis. Hyperkalemia lowers the transmembrane gradient in neurons, leading to neuronal depolarization independent of the degree of hyperkalemia. If the degree of hyperkalemia is large enough, this depolarization inactivates voltage-gated sodium channels, making neurons refractory to excitation. Electromyographical studies have shown reduction in the compounded muscle action potential.11 The transient nature of this paralysis is reflected by rapid correction of weakness and paralysis when the electrolyte disorder is corrected.
The patient in this case also presented with bradycardia. The ECG manifestations of hyperkalemia can include atrial asystole, intraventricular conduction disturbances, peaked T waves, and widened QRS complexes. However, some patients with renal insufficiency may not exhibit ECG changes despite significantly elevated serum potassium levels.12
The severity of hyperkalemia is crucial in determining the associated ECG changes, with levels > 6.0 mEq/L presenting with abnormalities.13 ECG findings alone may not always accurately reflect the severity of hyperkalemia, as up to 60% of patients with potassium levels > 6.0 mEq/L may not show ECG changes.14 Additionally, extreme hyperkalemia can lead to inconsistent ECG findings, making it challenging to rely solely on ECG for diagnosis and monitoring.8 The level of potassium that causes these effects varies widely through patient populations.
The main mechanism by which hyperkalemia affects the heart’s conduction system is through voltage differences across the conduction fibers and eventual steady-state inactivation of sodium channels. This combination of mechanisms shortens the action potential duration, allowing more cardiomyocytes to undergo synchronized depolarization. This amalgamation of cardiomyocytes repolarizing can be reflected on ECGs as peaked T waves. As the action potential decreases, there is a period during which cardiomyocytes are prone to tachyarrhythmias and ventricular fibrillation.
A reduced action potential may lead to increased rates of depolarization and thus conduction, which in some scenarios may increase heart rate. As the levels of potassium rise, intracellular accumulation impedes the entry of sodium by decreasing the cation gradient across the cell membrane. This effectively slows the sinus nodes and prolongs the QRS by slowing the overall propagation of action potentials. By this mechanism, conduction delays, blocks, or asystole are manifested. The patient in this case showed conduction delays, peaked T waves, and disappearance of P waves when he first arrived.
Hyperkalemia Treatment
Hyperkalemia develops most commonly due to acute or chronic kidney diseases, as was the case with this patient. The patient’s hyperkalemia was also augmented by the use of nonsteroidal anti-inflammatory drugs (NSAIDs), which can directly affect renal function. A properly functioning kidney is responsible for excretion of up to 90% of ingested potassium, while the remainder is excreted through the gastrointestinal (GI) tract. Definitive treatment of hyperkalemia is mitigated primarily through these 2 organ systems. The treatment also includes transitory mechanisms of potassium reduction. The goal of each method is to preserve the action potential of cardiomyocytes and myocytes. This patient presented with acute symptomatic hyperkalemia and received various medications to acutely, transitorily, and definitively treat it.
Initial therapy included calcium gluconate, which functions to stabilize the myocardial cell membrane. Hyperkalemia decreases the resting membrane action potential of excitable cells and predisposes them to early depolarization and thus dysrhythmias. Calcium decreases the threshold potential across cells and offsets the overall gradient back to near normal levels.15 Calcium can be delivered through calcium gluconate or calcium chloride. Calcium chloride is not preferred because extravasation can cause pain, blistering and tissue ischemia. Central venous access is required, potentially delaying prompt treatment. Calcium acts rapidly after administration—within 1 to 3 minutes—but only lasts 30 to 60 minutes.16 Administration of calcium gluconate can be repeated as often as necessary, but patients must be monitored for adverse effects of calcium such as nausea, abdominal pain, polydipsia, polyuria, muscle weakness, and paresthesia. Care must be taken when patients are taking digoxin, because calcium may potentiate toxicity.17 Although calcium provides immediate benefits it does little to correct the underlying cause; other medications are required to remove potassium from the body.
Two medication classes have been proven to shift potassium intracellularly. The first are β-2 agonists, such as albuterol/levalbuterol, and the second is insulin. Both work through sodium-potassium-ATPase in a direct manner. β-2 agonists stimulate sodium-potassium-ATPase to move more potassium intracellularly, but these effects have been seen only with high doses of albuterol, typically 4× the standard dose of 0.5 mg in nebulized solutions to achieve decreases in potassium of 0.3 to 0.6 mEq/L, although some trials have reported decreases of 0.62 to 0.98 mEq/L.15,18 These potassium-lowering effects of β-2 agonist are modest, but can be seen 20 to 30 minutes after administration and persist up to 1 to 2 hours. β-2 agonists are also readily affected by β blockers, which may reduce or negate the desired effect in hyperkalemia. For these reasons, a β-2 agonist should not be given as monotherapy and should be provided as an adjuvant to more independent therapies such as insulin. Insulin binds to receptors on muscle cells and increases the quantity of sodium-potassium-ATPase and glucose transporters. With this increase in influx pumps, surrounding tissues with higher resting membrane potentials can absorb the potassium load, thereby protecting cardiomyocytes.
Potassium Removal
Three methods are currently available to remove potassium from the body: GI excretion, renal excretion, and direct removal from the bloodstream. Under normal physiologic conditions, the kidneys account for about 90% of the body’s ability to remove potassium. Loop diuretics facilitate the removal of potassium by increasing urine production and have an additional potassium-wasting effect. Although the onset of action of loop diuretics is typically 30 to 60 minutes after oral administration, their effect can last for several hours. In this patient, furosemide was introduced later in the treatment plan to manage recurring hyperkalemia by enhancing renal potassium excretion.
Potassium binders such as patiromer act in the GI tract, effectively reducing serum potassium levels although with a slower onset of action than furosemide, generally taking hours to days to exert its effect. Both medications illustrate a tailored approach to managing potassium levels, adapted to the evolving needs and renal function of the patient. The last method is using hemodialysis—by far the most rapid method to remove potassium, but also the most invasive. The different methods of treating hyperkalemia are summarized in Table 2. This patient required multiple days of hemodialysis to completely correct the electrolyte disorder. Upon discharge, the patient continued oral furosemide 40 mg daily and eventually discontinued hemodialysis due to stable renal function.

Often, after correcting an inciting event, potassium stores in the body eventually stabilize and do not require additional follow-up. Patients prone to hyperkalemia should be thoroughly educated on medications to avoid (NSAIDs, ACEIs/ARBs, trimethoprim), an adequate low potassium diet, and symptoms that may warrant medical attention.19
Conclusions
This case illustrates the importance of recognizing the spectrum of manifestations of hyperkalemia, which ranged from muscle weakness to cardiac dysrhythmias. Management strategies for the patient included stabilization of cardiac membranes, potassium shifting, and potassium removal, each tailored to the patient’s individual clinical findings.
The case further illustrates the critical role of continuous monitoring and dynamic adjustment of therapeutic strategies in response to evolving clinical and laboratory findings. The initial and subsequent ECGs, alongside laboratory tests, were instrumental in guiding the adjustments needed in the treatment regimen, ensuring both the efficacy and safety of the interventions. This proactive approach can mitigate the risk of recurrent hyperkalemia and its complications.
Hyperkalemia involves elevated serum potassium levels (> 5.0 mEq/L) and represents an important electrolyte disturbance due to its potentially severe consequences, including cardiac effects that can lead to dysrhythmia and even asystole and death.1,2 In a US Medicare population, the prevalence of hyperkalemia has been estimated at 2.7% and is associated with substantial health care costs.3 The prevalence is even more marked in patients with preexisting conditions such as chronic kidney disease (CKD) and heart failure.4,5
Hyperkalemia can result from multiple factors, including impaired renal function, adrenal disease, adverse drug reactions of angiotensin-converting enzyme inhibitors (ACEIs) and other medications, and heritable mutations.6 Hyperkalemia poses a considerable clinical risk, associated with adverse outcomes such as myocardial infarction and increased mortality in patients with CKD.5,7,8 Electrocardiographic (ECG) changes associated with hyperkalemia play a vital role in guiding clinical decisions and treatment strategies.9 Understanding the pathophysiology, risk factors, and consequences of hyperkalemia, as well as the significance of ECG changes in its management, is essential for health care practitioners.
Case Presentation
An 81-year-old Hispanic man with a history of hypertension, hypothyroidism, gout, and CKD stage 3B presented to the emergency department with progressive weakness resulting in falls and culminating in an inability to ambulate independently. Additional symptoms included nausea, diarrhea, and myalgia. His vital signs were notable for a pulse of 41 beats/min. The physical examination was remarkable for significant weakness of the bilateral upper extremities, inability to bear his own weight, and bilateral lower extremity edema. His initial ECG upon arrival showed bradycardia with wide QRS, absent P waves, and peaked T waves (Figure 1a). These findings differed from his baseline ECG taken 1 year earlier, which showed sinus rhythm with premature atrial complexes and an old right bundle branch block (Figure 1b).

Medication review revealed that the patient was currently prescribed 100 mg allopurinol daily, 2.5 mg amlodipine daily, 10 mg atorvastatin at bedtime, 4 mg doxazosin daily, 112 mcg levothyroxine daily, 100 mg losartan daily, 25 mg metoprolol daily, and 0.4 mg tamsulosin daily. The patient had also been taking over-the-counter indomethacin for knee pain.
Based on the ECG results, he was treated with 0.083%/6 mL nebulized albuterol, 4.65 Mq/250 mL saline solution intravenous (IV) calcium gluconate, 10 units IV insulin with concomitant 50%/25 mL IV dextrose and 8.4 g of oral patiromer suspension. IV furosemide was held due to concern for renal function. The decision to proceed with hemodialysis was made. Repeat laboratory tests were performed, and an ECG obtained after treatment initiation but prior to hemodialysis demonstrated improvement of rate and T wave shortening (Figure 1c). The serum potassium level dropped from 9.8 mEq/L to 7.9 mEq/L (reference range, 3.5-5.0 mEq/L) (Table 1).

In addition to hemodialysis, sodium zirconium 10 g orally 3 times daily was added. Laboratory test results and an ECG was performed after dialysis continued to demonstrate improvement (Figure 1d). The patient’s potassium level decreased to 5.8 mEq/L, with the ECG demonstrating stability of heart rate and further improvement of the PR interval, QRS complex, and T waves.
Despite the established treatment regimen, potassium levels again rose to 6.7 mEq/L, but there were no significant changes in the ECG, and thus no medication changes were made (Figure 1e). Subsequent monitoring demonstrated a further increase in potassium to 7.4 mEq/L, with an ECG demonstrating a return to the baseline of 1 year prior. The patient underwent hemodialysis again and was given oral furosemide 60 mg every 12 hours. The potassium concentration after dialysis decreased to 4.7 mEq/L and remained stable, not going above 5.0 mEq/L on subsequent monitoring. The patient had resolution of all symptoms and was discharged.
Discussion
We have described in detail the presentation of each pathology and mechanisms of each treatment, starting with the patient’s initial condition that brought him to the emergency room—muscle weakness. Skeletal muscle weakness is a common manifestation of hyperkalemia, occurring in 20% to 40% of cases, and is more prevalent in severe elevations of potassium. Rarely, the weakness can progress to flaccid paralysis of the patient’s extremities and, in extreme cases, the diaphragm.
Muscle weakness progression occurs in a manner that resembles Guillain-Barré syndrome, starting in the lower extremities and ascending toward the upper extremities.10 This is known as secondary hyperkalemic periodic paralysis. Hyperkalemia lowers the transmembrane gradient in neurons, leading to neuronal depolarization independent of the degree of hyperkalemia. If the degree of hyperkalemia is large enough, this depolarization inactivates voltage-gated sodium channels, making neurons refractory to excitation. Electromyographical studies have shown reduction in the compounded muscle action potential.11 The transient nature of this paralysis is reflected by rapid correction of weakness and paralysis when the electrolyte disorder is corrected.
The patient in this case also presented with bradycardia. The ECG manifestations of hyperkalemia can include atrial asystole, intraventricular conduction disturbances, peaked T waves, and widened QRS complexes. However, some patients with renal insufficiency may not exhibit ECG changes despite significantly elevated serum potassium levels.12
The severity of hyperkalemia is crucial in determining the associated ECG changes, with levels > 6.0 mEq/L presenting with abnormalities.13 ECG findings alone may not always accurately reflect the severity of hyperkalemia, as up to 60% of patients with potassium levels > 6.0 mEq/L may not show ECG changes.14 Additionally, extreme hyperkalemia can lead to inconsistent ECG findings, making it challenging to rely solely on ECG for diagnosis and monitoring.8 The level of potassium that causes these effects varies widely through patient populations.
The main mechanism by which hyperkalemia affects the heart’s conduction system is through voltage differences across the conduction fibers and eventual steady-state inactivation of sodium channels. This combination of mechanisms shortens the action potential duration, allowing more cardiomyocytes to undergo synchronized depolarization. This amalgamation of cardiomyocytes repolarizing can be reflected on ECGs as peaked T waves. As the action potential decreases, there is a period during which cardiomyocytes are prone to tachyarrhythmias and ventricular fibrillation.
A reduced action potential may lead to increased rates of depolarization and thus conduction, which in some scenarios may increase heart rate. As the levels of potassium rise, intracellular accumulation impedes the entry of sodium by decreasing the cation gradient across the cell membrane. This effectively slows the sinus nodes and prolongs the QRS by slowing the overall propagation of action potentials. By this mechanism, conduction delays, blocks, or asystole are manifested. The patient in this case showed conduction delays, peaked T waves, and disappearance of P waves when he first arrived.
Hyperkalemia Treatment
Hyperkalemia develops most commonly due to acute or chronic kidney diseases, as was the case with this patient. The patient’s hyperkalemia was also augmented by the use of nonsteroidal anti-inflammatory drugs (NSAIDs), which can directly affect renal function. A properly functioning kidney is responsible for excretion of up to 90% of ingested potassium, while the remainder is excreted through the gastrointestinal (GI) tract. Definitive treatment of hyperkalemia is mitigated primarily through these 2 organ systems. The treatment also includes transitory mechanisms of potassium reduction. The goal of each method is to preserve the action potential of cardiomyocytes and myocytes. This patient presented with acute symptomatic hyperkalemia and received various medications to acutely, transitorily, and definitively treat it.
Initial therapy included calcium gluconate, which functions to stabilize the myocardial cell membrane. Hyperkalemia decreases the resting membrane action potential of excitable cells and predisposes them to early depolarization and thus dysrhythmias. Calcium decreases the threshold potential across cells and offsets the overall gradient back to near normal levels.15 Calcium can be delivered through calcium gluconate or calcium chloride. Calcium chloride is not preferred because extravasation can cause pain, blistering and tissue ischemia. Central venous access is required, potentially delaying prompt treatment. Calcium acts rapidly after administration—within 1 to 3 minutes—but only lasts 30 to 60 minutes.16 Administration of calcium gluconate can be repeated as often as necessary, but patients must be monitored for adverse effects of calcium such as nausea, abdominal pain, polydipsia, polyuria, muscle weakness, and paresthesia. Care must be taken when patients are taking digoxin, because calcium may potentiate toxicity.17 Although calcium provides immediate benefits it does little to correct the underlying cause; other medications are required to remove potassium from the body.
Two medication classes have been proven to shift potassium intracellularly. The first are β-2 agonists, such as albuterol/levalbuterol, and the second is insulin. Both work through sodium-potassium-ATPase in a direct manner. β-2 agonists stimulate sodium-potassium-ATPase to move more potassium intracellularly, but these effects have been seen only with high doses of albuterol, typically 4× the standard dose of 0.5 mg in nebulized solutions to achieve decreases in potassium of 0.3 to 0.6 mEq/L, although some trials have reported decreases of 0.62 to 0.98 mEq/L.15,18 These potassium-lowering effects of β-2 agonist are modest, but can be seen 20 to 30 minutes after administration and persist up to 1 to 2 hours. β-2 agonists are also readily affected by β blockers, which may reduce or negate the desired effect in hyperkalemia. For these reasons, a β-2 agonist should not be given as monotherapy and should be provided as an adjuvant to more independent therapies such as insulin. Insulin binds to receptors on muscle cells and increases the quantity of sodium-potassium-ATPase and glucose transporters. With this increase in influx pumps, surrounding tissues with higher resting membrane potentials can absorb the potassium load, thereby protecting cardiomyocytes.
Potassium Removal
Three methods are currently available to remove potassium from the body: GI excretion, renal excretion, and direct removal from the bloodstream. Under normal physiologic conditions, the kidneys account for about 90% of the body’s ability to remove potassium. Loop diuretics facilitate the removal of potassium by increasing urine production and have an additional potassium-wasting effect. Although the onset of action of loop diuretics is typically 30 to 60 minutes after oral administration, their effect can last for several hours. In this patient, furosemide was introduced later in the treatment plan to manage recurring hyperkalemia by enhancing renal potassium excretion.
Potassium binders such as patiromer act in the GI tract, effectively reducing serum potassium levels although with a slower onset of action than furosemide, generally taking hours to days to exert its effect. Both medications illustrate a tailored approach to managing potassium levels, adapted to the evolving needs and renal function of the patient. The last method is using hemodialysis—by far the most rapid method to remove potassium, but also the most invasive. The different methods of treating hyperkalemia are summarized in Table 2. This patient required multiple days of hemodialysis to completely correct the electrolyte disorder. Upon discharge, the patient continued oral furosemide 40 mg daily and eventually discontinued hemodialysis due to stable renal function.

Often, after correcting an inciting event, potassium stores in the body eventually stabilize and do not require additional follow-up. Patients prone to hyperkalemia should be thoroughly educated on medications to avoid (NSAIDs, ACEIs/ARBs, trimethoprim), an adequate low potassium diet, and symptoms that may warrant medical attention.19
Conclusions
This case illustrates the importance of recognizing the spectrum of manifestations of hyperkalemia, which ranged from muscle weakness to cardiac dysrhythmias. Management strategies for the patient included stabilization of cardiac membranes, potassium shifting, and potassium removal, each tailored to the patient’s individual clinical findings.
The case further illustrates the critical role of continuous monitoring and dynamic adjustment of therapeutic strategies in response to evolving clinical and laboratory findings. The initial and subsequent ECGs, alongside laboratory tests, were instrumental in guiding the adjustments needed in the treatment regimen, ensuring both the efficacy and safety of the interventions. This proactive approach can mitigate the risk of recurrent hyperkalemia and its complications.
- Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381-401. doi:10.1146/annurev.physiol.010908.163241 2.
- Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. In: StatPearls. StatPearls Publishing; September 4, 2023. Accessed October 22, 2025.
- Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36:1333-1341. doi:10.1080/03007995.2020.1775072
- Loutradis C, Tolika P, Skodra A, et al. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested case-control study. Am J Nephrol. 2015;42:351-360. doi:10.1159/000442393
- Grodzinsky A, Goyal A, Gosch K, et al. Prevalence and prognosis of hyperkalemia in patients with acute myocardial infarction. Am J Med. 2016;129:858-865. doi:10.1016/j.amjmed.2016.03.008
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(suppl 3):iii2-iii11. doi:10.1093/ndt/gfz206
- Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90-100. doi:10.2215/CJN.01730215
- Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28:3155-3165. doi:10.1681/ASN.2016121344
- Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721-729. doi:10.1053/ajem.2000.7344
- Kimmons LA, Usery JB. Acute ascending muscle weakness secondary to medication-induced hyperkalemia. Case Rep Med. 2014;2014:789529. doi:10.1155/2014/789529
- Naik KR, Saroja AO, Khanpet MS. Reversible electrophysiological abnormalities in acute secondary hyperkalemic paralysis. Ann Indian Acad Neurol. 2012;15:339-343. doi:10.4103/0972-2327.104354
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324-330. doi:10.2215/CJN.04611007
- Larivée NL, Michaud JB, More KM, Wilson JA, Tennankore KK. Hyperkalemia: prevalence, predictors and emerging treatments. Cardiol Ther. 2023;12:35-63. doi:10.1007/s40119-022-00289-z
- Shingarev R, Allon M. A physiologic-based approach to the treatment of acute hyperkalemia. Am J Kidney Dis. 2010;56:578-584. doi:10.1053/j.ajkd.2010.03.014
- Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40-47.
- Ng KE, Lee CS. Updated treatment options in the management of hyperkalemia. U.S. Pharmacist. February 16, 2017. Accessed October 1, 2025. www.uspharmacist.com/article/updated-treatment-options-in-the-management-of-hyperkalemia
- Quick G, Bastani B. Prolonged asystolic hyperkalemic cardiac arrest with no neurologic sequelae. Ann Emerg Med. 1994;24:305-311. doi:10.1016/s0196-0644(94)70144-x 18.
- Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med. 1989;110:426-429. doi:10.7326/0003-4819-110-6-42619.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4 suppl):S117-S314. doi:10.1016/j.kint.2023.10.018
- Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381-401. doi:10.1146/annurev.physiol.010908.163241 2.
- Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. In: StatPearls. StatPearls Publishing; September 4, 2023. Accessed October 22, 2025.
- Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36:1333-1341. doi:10.1080/03007995.2020.1775072
- Loutradis C, Tolika P, Skodra A, et al. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested case-control study. Am J Nephrol. 2015;42:351-360. doi:10.1159/000442393
- Grodzinsky A, Goyal A, Gosch K, et al. Prevalence and prognosis of hyperkalemia in patients with acute myocardial infarction. Am J Med. 2016;129:858-865. doi:10.1016/j.amjmed.2016.03.008
- Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(suppl 3):iii2-iii11. doi:10.1093/ndt/gfz206
- Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90-100. doi:10.2215/CJN.01730215
- Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28:3155-3165. doi:10.1681/ASN.2016121344
- Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721-729. doi:10.1053/ajem.2000.7344
- Kimmons LA, Usery JB. Acute ascending muscle weakness secondary to medication-induced hyperkalemia. Case Rep Med. 2014;2014:789529. doi:10.1155/2014/789529
- Naik KR, Saroja AO, Khanpet MS. Reversible electrophysiological abnormalities in acute secondary hyperkalemic paralysis. Ann Indian Acad Neurol. 2012;15:339-343. doi:10.4103/0972-2327.104354
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324-330. doi:10.2215/CJN.04611007
- Larivée NL, Michaud JB, More KM, Wilson JA, Tennankore KK. Hyperkalemia: prevalence, predictors and emerging treatments. Cardiol Ther. 2023;12:35-63. doi:10.1007/s40119-022-00289-z
- Shingarev R, Allon M. A physiologic-based approach to the treatment of acute hyperkalemia. Am J Kidney Dis. 2010;56:578-584. doi:10.1053/j.ajkd.2010.03.014
- Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40-47.
- Ng KE, Lee CS. Updated treatment options in the management of hyperkalemia. U.S. Pharmacist. February 16, 2017. Accessed October 1, 2025. www.uspharmacist.com/article/updated-treatment-options-in-the-management-of-hyperkalemia
- Quick G, Bastani B. Prolonged asystolic hyperkalemic cardiac arrest with no neurologic sequelae. Ann Emerg Med. 1994;24:305-311. doi:10.1016/s0196-0644(94)70144-x 18.
- Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med. 1989;110:426-429. doi:10.7326/0003-4819-110-6-42619.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4 suppl):S117-S314. doi:10.1016/j.kint.2023.10.018
Following the Hyperkalemia Trail: A Case Report of ECG Changes and Treatment Responses
Following the Hyperkalemia Trail: A Case Report of ECG Changes and Treatment Responses
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.
When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).
Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Targeted Syncope Workup in Hypercoagulable Patients
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Hypereosinophilic Syndrome With Eosinophilic Endomyocarditis: A Rare Cardiac Manifestation
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
The Immune Heartache: Pericarditis Following Checkpoint Inhibition
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology