User login
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
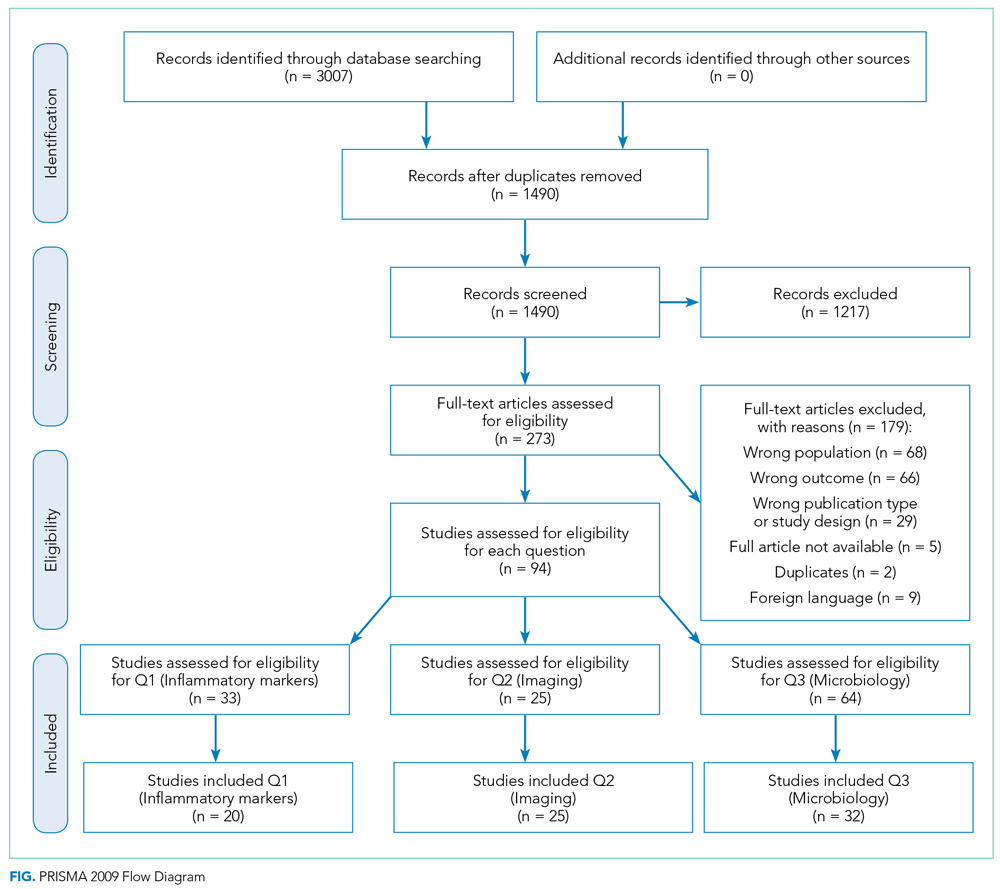
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.
Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
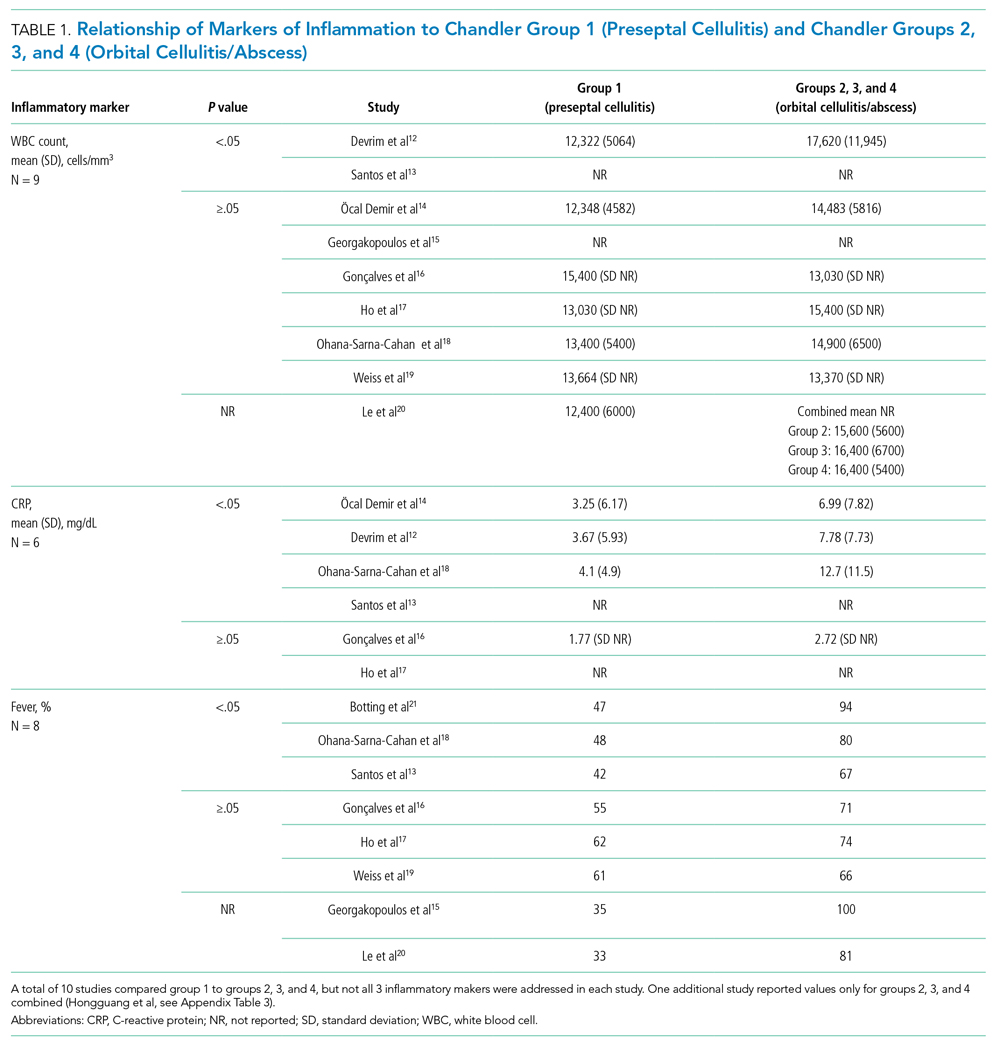
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).
Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
© 2021 Society of Hospital Medicine