User login
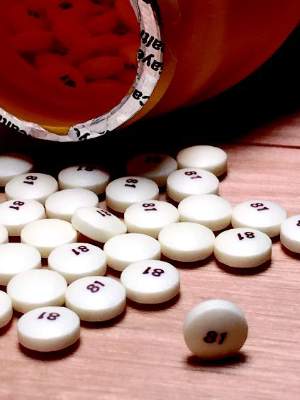
VIENNA – Postdiagnosis aspirin use nearly doubled survival in patients with gastrointestinal cancers in a large study from the Netherlands.
After 5 years, 75% of daily low-dose–aspirin users were alive, compared with 42% of patients who were nonusers.
The beneficial effect of aspirin was seen for every gastrointestinal (GI) cancer (esophageal, stomach, hepatobiliary tract, colon, and rectal), except pancreatic cancer, Dr. Martine Frouws of Leiden (the Netherlands) University Medical Center reported at the European Society for Medical Oncology congress.
Many studies have suggested a role for regular aspirin use in reducing cancer incidence and mortality, particularly colorectal cancer, but this is the first time that survival data have been analyzed from patients with tumors in different GI locations.
“If aspirin can become a regular treatment for cancer, this can have a large impact on cancer survival and global health,” Dr. Frouws said in a press briefing.
The retrospective study involved 13,715 patients who had been diagnosed with a GI cancer between 1998 and 2011 in the population-based Eindhoven Cancer Registry and were linked to pharmacy data from PHARMO, the Institute for Drug Outcomes Research based in Utrecht.
Of these, 4,187 (30.5%) used aspirin before their cancer diagnosis, 1,143 (8.3%) were solely postdiagnosis users, and 8,385 (61.1%) did not take aspirin at all. Patients with prediagnosis aspirin use were excluded from the analysis.
Nearly half of the patients had colon cancer (43%), but a significant portion had rectal cancer (25%) and esophageal cancer (10.2%).
Median follow-up for all patients was 48.6 months, with 28% of patients surviving for at least 5 years.
The survival benefit with aspirin remained in patients with GI tumors after adjusting for sex, age, stage of cancer, surgery, radiotherapy, chemotherapy, and other medical conditions, Dr. Frouws reported.
“Medical research is focusing more and more on personalized medicine, but many personalized treatments are expensive and only useful in small populations,” she said in a statement. “We believe that our research shows quite the opposite – it demonstrates the considerable benefit of a cheap, well-established, and easily obtainable drug in a larger group of patients, while still targeting the treatment to a specific individual.”
Dr. Peter Naredi, scientific cochair of the cancer congress, who was not involved in the study, commented, “We have good evidence that the frequent use of aspirin in the population can prevent some cases of colorectal cancer. Now, Dr. Frouws and colleagues show that, in over 13,000 patients diagnosed with a gastrointestinal cancer, aspirin also improved survival compared with those who did not use it. With more and more data to support the beneficial role of aspirin, we must consider whether we should recommend it to a wider public.”
In its recent draft recommendations, the U.S. Preventive Services Task Force “recommends low-dose aspirin use for the primary prevention of cardiovascular disease and colorectal cancer in adults ages 50 to 59 years” who meet certain requirements and suggests aspirin use be individualized for those aged 60-69 years.
The authors and other researchers theorize that the antiplatelet effect of aspirin plays a key role in its anticancer effects. Circulating tumor cells are thought to hide from the immune system with the help of the platelets that surround them. Aspirin inhibits platelet function and therefore allows the immune system to better recognize and eliminate circulating tumor cells, Dr. Frouws explained.
The next step is to study the characteristics of tumors in patients for whom aspirin was beneficial to identify those who could profit from aspirin treatment in the future.
Because the study provides no information on the side effects of aspirin when used as a treatment for cancer, randomized trials have to be done to confirm the effect of aspirin on cancer, she said.
A slew of trials is currently underway to do just that, including the phase III, placebo-controlled Aspirin trial in the Netherlands studying the effects of aspirin 80 mg once daily on recurrence and survival in elderly patients with stage II and III colon cancer.
Other aspirin trials include the U.S. and Australian ASPREE trial in patients older than 65 years, CaPP3 in patients with Lynch syndrome, and the Asian ASCOLT trial for patients with Dukes C and high-risk Dukes B colorectal cancers.
In September, researchers at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital received a $10 million award to study the impact of aspirin on breast cancer recurrence and mortality in the Aspirin for Breast Cancer (ABC) trial.
“Aspirin may serve as the magic bullet because it can target and prevent ischemic heart disease, cancer, and Alzheimer’s disease, the three major health catastrophes in the third millennium,” Dr. Nadir Arber, director of the Integrated Cancer Prevention Center, Tel Aviv Sourasky Medical Center, who was not involved in the research, said in a statement.
On Twitter @pwendl
VIENNA – Postdiagnosis aspirin use nearly doubled survival in patients with gastrointestinal cancers in a large study from the Netherlands.
After 5 years, 75% of daily low-dose–aspirin users were alive, compared with 42% of patients who were nonusers.
The beneficial effect of aspirin was seen for every gastrointestinal (GI) cancer (esophageal, stomach, hepatobiliary tract, colon, and rectal), except pancreatic cancer, Dr. Martine Frouws of Leiden (the Netherlands) University Medical Center reported at the European Society for Medical Oncology congress.
Many studies have suggested a role for regular aspirin use in reducing cancer incidence and mortality, particularly colorectal cancer, but this is the first time that survival data have been analyzed from patients with tumors in different GI locations.
“If aspirin can become a regular treatment for cancer, this can have a large impact on cancer survival and global health,” Dr. Frouws said in a press briefing.
The retrospective study involved 13,715 patients who had been diagnosed with a GI cancer between 1998 and 2011 in the population-based Eindhoven Cancer Registry and were linked to pharmacy data from PHARMO, the Institute for Drug Outcomes Research based in Utrecht.
Of these, 4,187 (30.5%) used aspirin before their cancer diagnosis, 1,143 (8.3%) were solely postdiagnosis users, and 8,385 (61.1%) did not take aspirin at all. Patients with prediagnosis aspirin use were excluded from the analysis.
Nearly half of the patients had colon cancer (43%), but a significant portion had rectal cancer (25%) and esophageal cancer (10.2%).
Median follow-up for all patients was 48.6 months, with 28% of patients surviving for at least 5 years.
The survival benefit with aspirin remained in patients with GI tumors after adjusting for sex, age, stage of cancer, surgery, radiotherapy, chemotherapy, and other medical conditions, Dr. Frouws reported.
“Medical research is focusing more and more on personalized medicine, but many personalized treatments are expensive and only useful in small populations,” she said in a statement. “We believe that our research shows quite the opposite – it demonstrates the considerable benefit of a cheap, well-established, and easily obtainable drug in a larger group of patients, while still targeting the treatment to a specific individual.”
Dr. Peter Naredi, scientific cochair of the cancer congress, who was not involved in the study, commented, “We have good evidence that the frequent use of aspirin in the population can prevent some cases of colorectal cancer. Now, Dr. Frouws and colleagues show that, in over 13,000 patients diagnosed with a gastrointestinal cancer, aspirin also improved survival compared with those who did not use it. With more and more data to support the beneficial role of aspirin, we must consider whether we should recommend it to a wider public.”
In its recent draft recommendations, the U.S. Preventive Services Task Force “recommends low-dose aspirin use for the primary prevention of cardiovascular disease and colorectal cancer in adults ages 50 to 59 years” who meet certain requirements and suggests aspirin use be individualized for those aged 60-69 years.
The authors and other researchers theorize that the antiplatelet effect of aspirin plays a key role in its anticancer effects. Circulating tumor cells are thought to hide from the immune system with the help of the platelets that surround them. Aspirin inhibits platelet function and therefore allows the immune system to better recognize and eliminate circulating tumor cells, Dr. Frouws explained.
The next step is to study the characteristics of tumors in patients for whom aspirin was beneficial to identify those who could profit from aspirin treatment in the future.
Because the study provides no information on the side effects of aspirin when used as a treatment for cancer, randomized trials have to be done to confirm the effect of aspirin on cancer, she said.
A slew of trials is currently underway to do just that, including the phase III, placebo-controlled Aspirin trial in the Netherlands studying the effects of aspirin 80 mg once daily on recurrence and survival in elderly patients with stage II and III colon cancer.
Other aspirin trials include the U.S. and Australian ASPREE trial in patients older than 65 years, CaPP3 in patients with Lynch syndrome, and the Asian ASCOLT trial for patients with Dukes C and high-risk Dukes B colorectal cancers.
In September, researchers at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital received a $10 million award to study the impact of aspirin on breast cancer recurrence and mortality in the Aspirin for Breast Cancer (ABC) trial.
“Aspirin may serve as the magic bullet because it can target and prevent ischemic heart disease, cancer, and Alzheimer’s disease, the three major health catastrophes in the third millennium,” Dr. Nadir Arber, director of the Integrated Cancer Prevention Center, Tel Aviv Sourasky Medical Center, who was not involved in the research, said in a statement.
On Twitter @pwendl
VIENNA – Postdiagnosis aspirin use nearly doubled survival in patients with gastrointestinal cancers in a large study from the Netherlands.
After 5 years, 75% of daily low-dose–aspirin users were alive, compared with 42% of patients who were nonusers.
The beneficial effect of aspirin was seen for every gastrointestinal (GI) cancer (esophageal, stomach, hepatobiliary tract, colon, and rectal), except pancreatic cancer, Dr. Martine Frouws of Leiden (the Netherlands) University Medical Center reported at the European Society for Medical Oncology congress.
Many studies have suggested a role for regular aspirin use in reducing cancer incidence and mortality, particularly colorectal cancer, but this is the first time that survival data have been analyzed from patients with tumors in different GI locations.
“If aspirin can become a regular treatment for cancer, this can have a large impact on cancer survival and global health,” Dr. Frouws said in a press briefing.
The retrospective study involved 13,715 patients who had been diagnosed with a GI cancer between 1998 and 2011 in the population-based Eindhoven Cancer Registry and were linked to pharmacy data from PHARMO, the Institute for Drug Outcomes Research based in Utrecht.
Of these, 4,187 (30.5%) used aspirin before their cancer diagnosis, 1,143 (8.3%) were solely postdiagnosis users, and 8,385 (61.1%) did not take aspirin at all. Patients with prediagnosis aspirin use were excluded from the analysis.
Nearly half of the patients had colon cancer (43%), but a significant portion had rectal cancer (25%) and esophageal cancer (10.2%).
Median follow-up for all patients was 48.6 months, with 28% of patients surviving for at least 5 years.
The survival benefit with aspirin remained in patients with GI tumors after adjusting for sex, age, stage of cancer, surgery, radiotherapy, chemotherapy, and other medical conditions, Dr. Frouws reported.
“Medical research is focusing more and more on personalized medicine, but many personalized treatments are expensive and only useful in small populations,” she said in a statement. “We believe that our research shows quite the opposite – it demonstrates the considerable benefit of a cheap, well-established, and easily obtainable drug in a larger group of patients, while still targeting the treatment to a specific individual.”
Dr. Peter Naredi, scientific cochair of the cancer congress, who was not involved in the study, commented, “We have good evidence that the frequent use of aspirin in the population can prevent some cases of colorectal cancer. Now, Dr. Frouws and colleagues show that, in over 13,000 patients diagnosed with a gastrointestinal cancer, aspirin also improved survival compared with those who did not use it. With more and more data to support the beneficial role of aspirin, we must consider whether we should recommend it to a wider public.”
In its recent draft recommendations, the U.S. Preventive Services Task Force “recommends low-dose aspirin use for the primary prevention of cardiovascular disease and colorectal cancer in adults ages 50 to 59 years” who meet certain requirements and suggests aspirin use be individualized for those aged 60-69 years.
The authors and other researchers theorize that the antiplatelet effect of aspirin plays a key role in its anticancer effects. Circulating tumor cells are thought to hide from the immune system with the help of the platelets that surround them. Aspirin inhibits platelet function and therefore allows the immune system to better recognize and eliminate circulating tumor cells, Dr. Frouws explained.
The next step is to study the characteristics of tumors in patients for whom aspirin was beneficial to identify those who could profit from aspirin treatment in the future.
Because the study provides no information on the side effects of aspirin when used as a treatment for cancer, randomized trials have to be done to confirm the effect of aspirin on cancer, she said.
A slew of trials is currently underway to do just that, including the phase III, placebo-controlled Aspirin trial in the Netherlands studying the effects of aspirin 80 mg once daily on recurrence and survival in elderly patients with stage II and III colon cancer.
Other aspirin trials include the U.S. and Australian ASPREE trial in patients older than 65 years, CaPP3 in patients with Lynch syndrome, and the Asian ASCOLT trial for patients with Dukes C and high-risk Dukes B colorectal cancers.
In September, researchers at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital received a $10 million award to study the impact of aspirin on breast cancer recurrence and mortality in the Aspirin for Breast Cancer (ABC) trial.
“Aspirin may serve as the magic bullet because it can target and prevent ischemic heart disease, cancer, and Alzheimer’s disease, the three major health catastrophes in the third millennium,” Dr. Nadir Arber, director of the Integrated Cancer Prevention Center, Tel Aviv Sourasky Medical Center, who was not involved in the research, said in a statement.
On Twitter @pwendl
AT THE EUROPEAN CANCER CONGRESS 2015
Key clinical point: Aspirin use initiated after diagnosis of gastrointestinal cancers is associated with higher survival rates.
Major finding: Five-year survival was 75% in postdiagnosis aspirin users vs. 42% in nonusers.
Data source: Retrospective study involving 13,715 patients with gastrointestinal tumors.
Disclosures: The authors reported having no significant disclosures.