User login
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
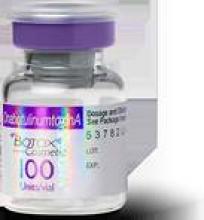
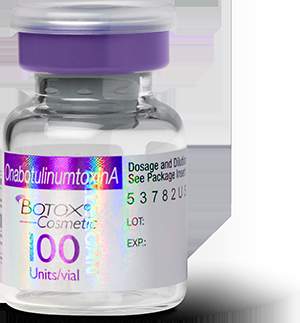
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at [email protected].
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at [email protected].
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at [email protected].
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
FROM THE FDA