User login
- Don’t overestimate the value of a rapid influenza test. The sensitivity of these tests ranges between 10% and 70% in 2009 H1N1 influenza infection.
- Provide chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection.
- Consider longer courses of oseltamivir (beyond the standard 75 mg twice daily for 5 days) among hospitalized patients.
CASE: A 28-year-old woman (G6P1) at 33 weeks’ gestation was transferred from an outside hospital with worsening tachypnea, increasing oxygen requirement, and worsening infiltrates on chest radiograph.
A week earlier she had presented to a local emergency department (ED) with a 1-day history of nonproductive cough, fever, congestion, and decreased fetal movement. She also complained of vomiting. Examination was notable for an oxygen saturation of 99% on room air, heart rate of 126 bpm, temperature of 37.9°C (100.2°F), and blood pressure (BP) of 104/70 mm Hg. Rapid influenza A/B nasopharyngeal swab and group A Streptococcus direct probe were both negative. She was transferred to labor and delivery for fetal monitoring and discharged later that day.
Later in the week she returned to 2 other hospitals due to continued symptoms. She was diagnosed with right upper lobe pneumonia on her third ED visit and transferred to our facility, with increasing respiratory distress. Her examination was notable for a temperature of 36.8°C (98.2°F), pulse of 103 bpm, BP of 98/56 mm Hg, respiratory rate of 27 breaths per minute, and oxygen saturation of 94%. The patient had ulcerations on her tongue, dry mucous membranes, and lower extremity edema; on lung exam she had right lower lobe crackles and occasional wheezes.
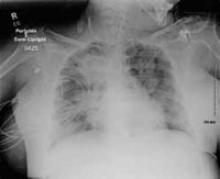
Lab results were notable for a serum hemoglobin of 9.3 g/dL and platelet count of 75,000/mm3. The leukocyte count was 8.3×109/L, with differential remarkable for 24% bands. Potassium was 3.3 mEq/L and bicarbonate was 19 mEq/L; the basic metabolic panel was otherwise normal. Lactic acid was elevated at 2.4 mg/dL. Coagulation levels were normal. Urinalysis was negative. Chest radiograph (FIGURE) was read as “right upper lobe pneumonia and probable small bilateral pleural effusions with lower lung airspace disease, which may relate to atelectasis; however, superimposed multi-focal pneumonia is not excluded.”
Overnight, she had an increasing oxygen requirement of up to 15 liters, axillary temperature 40.8°C (105.6°F), and heart rate in the 140s; fetal heart rate was in the 200s. The next day, a chest x-ray revealed worsening pulmonary infiltrates. The patient was tachypneic, with a BP of 99/50 mm Hg. She continued to worsen and required intubation for hypoxic respiratory distress.
FIGURE
Right upper lobe pneumonia
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
H1N1 pneumonia
The patient’s physician initiated broad-spectrum antibiotics and oseltamivir for a presumptive diagnosis of 2009 H1N1 pneumonia and possible aspiration pneumonia. Although the patient had negative rapid influenza tests, the sensitivity of these tests is 10% to 70% in 2009 H1N1 influenza infection.1
Pregnant women and those in the first 2 weeks postpartum (or who have experienced a pregnancy loss) are considered to be at high risk for complications of influenza infection.1 Influenza A infection in pregnancy is associated with preterm labor, preterm birth, pneumonia, acute respiratory distress syndrome, and death.2 Although many pregnant patients may present with mild or moderate symptoms, the clinical progression with 2009 H1N1 appears to be more rapid than what has been seen with previous seasonal influenza outbreaks.3
According to 1 study, hospital admission rates during the first month of the outbreak were higher for pregnant women compared with the general population: 0.32 vs 0.076 per 100,000.4 The Centers for Disease Control and Prevention (CDC) indicates that while 1% of the population is pregnant at any given time, 6% of confirmed deaths from H1N1 in the United States in 2009 were pregnant women.5 Two prospective observational studies published in the Journal of the American Medical Association revealed the percentages of critically ill H1N1 patients who were pregnant. In Canada, 7.7% of critically ill patients with H1N1 were pregnant. In California, 10% were pregnant, and 6% of fatal cases in patients over age 18 were pregnant women.6,7
Why are pregnant women more susceptible to flu complications?
The immune system changes that make pregnant women more susceptible to complications of influenza infection are not well understood. Normal physiologic changes to the respiratory system during pregnancy may be a contributing factor. These include increased minute ventilation in the first trimester due to an increase in progesterone levels, increased tidal volume, decreased residual volume and functional residual capacity due to the mechanical effect of a gravid uterus, and increased oxygen consumption and basal metabolic rate due to increased demand.8
What can be done to decrease their risk?
The first step is preventing infection. For the 2009-2010 season, vaccination against seasonal and 2009 H1N1 influenza is strongly recommended for all pregnant women. Only the intramuscular injection is approved for pregnant women. Patients can receive the seasonal influenza vaccine at the same time as the H1N1 vaccine using an alternate injection site.
Maternal immunization against seasonal influenza benefits mothers and has also been shown to lower infection rates in infants. A study published in The New England Journal of Medicine showed that the seasonal influenza vaccination given to pregnant women reduced influenza-like illness in their infants younger than 6 months of age by 63%.9 Also important is providing chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection. For 2009 H1N1 chemoprophylaxis, a 10-day course of once-daily oseltamivir or zanamivir is acceptable. Zanamivir is an inhaled medication and should not be prescribed to patients with asthma or other respiratory conditions.
Confirmed case? Tx for the pregnant patient
The 2009 H1N1 virus is susceptible to oseltamivir and zanamivir.1 Both antivirals are Category C in pregnancy. The CDC recommends that patients with suspected or confirmed 2009 H1N1 infection who are in high-risk groups (which includes pregnant women) be treated with oseltamivir.
Antiviral medications such as oseltamivir and zanamivir act at the viral replication stage, which peaks at 24 to 72 hours in influenza.10 This helps explain evidence that the earlier treatment of influenza is initiated—within the first 48 hours—the more effective it is in reducing fever, relieving symptoms, and decreasing time to return to baseline activity.11 A study of pregnant women in California with severe 2009 H1N1 infection found that later treatment (>2 days after symptom onset) was associated with 4 times the risk of admission and death.3 For these reasons, treatment should not be delayed while test results are pending.
That said, in hospitalized patients with seasonal influenza, initiating treatment after 48 hours of symptom onset has been shown to provide some benefit in some observational studies.1,12 Consequently, the CDC recommends initiating treatment of high-risk patients who seek care more than 48 hours after symptom onset.1
The standard course of oseltamivir is 75 mg twice daily for 5 days. Longer courses may be beneficial in hospitalized patients.1 Oseltamivir and zanamivir can also be continued while breastfeeding.13
Our patient’s outcome
The patient received a 10-day course of oseltamivir (rather than the standard 5-day course), as well as empiric broad-spectrum antibiotic coverage for community-acquired pneumonia and aspiration pneumonia, including coverage for Streptococcus pneumoniae (the most common bacterial cause of secondary pneumonia in influenza14).
The cultures come back. Nasopharyngeal cultures were negative × 2 for type A influenza. Blood cultures were negative throughout the admission. Sputum cultures were negative, as well. Bronchoscopy cultures, however, were positive for type A influenza and negative for bacterial and fungal pathogens, confirming a diagnosis of primary pneumonia from 2009 H1N1 infection.
The patient was extubated 1 week after her arrival at our hospital and continued to recover during the rest of her hospital stay. She was discharged in stable condition. Several weeks later, she delivered a full-term infant with average weight and normal Apgar scores.
CORRESPONDENCE: Christopher Bernheisel, MD, Director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave., Suite 340, Cincinnati, OH 45219; [email protected]
1. Centers for Disease Control and Prevention. Updated interim recommendations for obstetric health care providers related to use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/pregnancy/antiviral_messages.htm. Accessed January 9, 2010.
2. Saleeby E, Chapman J, Morse J, et al. H1N1 Influenza in pregnancy: cause for concern. Obstet Gynecol. 2009;114:885-891.
3. Louie J, Acosta M, Jamieson D, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27-35.
4. Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
5. Centers for Disease Control and Prevention. 2009 H1N1 influenza vaccine and pregnant women: information for healthcare providers. Available at: http://www.cdc.gov/h1n1flu/vaccination/providers_qa.htm. Accessed January 9, 2010.
6. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872-1879.
7. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302:1896-1902.
8. Ratcliffe SD, Baxley EG, Cline MK, et al. Family Medicine Obstetrics. 3rd ed. Philadelphia, Pa: Mosby/Elsevier; 2008:203.
9. Zaman K, Roy E, Arifeen S, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555-1564.
10. Moscana A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363-1373.
11. Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123-129.
12. Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med. 2009;361:e110.-
13. United States National Library of Medicine. LactMed: drugs and lactation database. Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT. Accessed December 2, 2009.
14. Centers for Disease Control and Prevention. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325-329.
- Don’t overestimate the value of a rapid influenza test. The sensitivity of these tests ranges between 10% and 70% in 2009 H1N1 influenza infection.
- Provide chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection.
- Consider longer courses of oseltamivir (beyond the standard 75 mg twice daily for 5 days) among hospitalized patients.
CASE: A 28-year-old woman (G6P1) at 33 weeks’ gestation was transferred from an outside hospital with worsening tachypnea, increasing oxygen requirement, and worsening infiltrates on chest radiograph.
A week earlier she had presented to a local emergency department (ED) with a 1-day history of nonproductive cough, fever, congestion, and decreased fetal movement. She also complained of vomiting. Examination was notable for an oxygen saturation of 99% on room air, heart rate of 126 bpm, temperature of 37.9°C (100.2°F), and blood pressure (BP) of 104/70 mm Hg. Rapid influenza A/B nasopharyngeal swab and group A Streptococcus direct probe were both negative. She was transferred to labor and delivery for fetal monitoring and discharged later that day.
Later in the week she returned to 2 other hospitals due to continued symptoms. She was diagnosed with right upper lobe pneumonia on her third ED visit and transferred to our facility, with increasing respiratory distress. Her examination was notable for a temperature of 36.8°C (98.2°F), pulse of 103 bpm, BP of 98/56 mm Hg, respiratory rate of 27 breaths per minute, and oxygen saturation of 94%. The patient had ulcerations on her tongue, dry mucous membranes, and lower extremity edema; on lung exam she had right lower lobe crackles and occasional wheezes.
Lab results were notable for a serum hemoglobin of 9.3 g/dL and platelet count of 75,000/mm3. The leukocyte count was 8.3×109/L, with differential remarkable for 24% bands. Potassium was 3.3 mEq/L and bicarbonate was 19 mEq/L; the basic metabolic panel was otherwise normal. Lactic acid was elevated at 2.4 mg/dL. Coagulation levels were normal. Urinalysis was negative. Chest radiograph (FIGURE) was read as “right upper lobe pneumonia and probable small bilateral pleural effusions with lower lung airspace disease, which may relate to atelectasis; however, superimposed multi-focal pneumonia is not excluded.”
Overnight, she had an increasing oxygen requirement of up to 15 liters, axillary temperature 40.8°C (105.6°F), and heart rate in the 140s; fetal heart rate was in the 200s. The next day, a chest x-ray revealed worsening pulmonary infiltrates. The patient was tachypneic, with a BP of 99/50 mm Hg. She continued to worsen and required intubation for hypoxic respiratory distress.
FIGURE
Right upper lobe pneumonia
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
H1N1 pneumonia
The patient’s physician initiated broad-spectrum antibiotics and oseltamivir for a presumptive diagnosis of 2009 H1N1 pneumonia and possible aspiration pneumonia. Although the patient had negative rapid influenza tests, the sensitivity of these tests is 10% to 70% in 2009 H1N1 influenza infection.1
Pregnant women and those in the first 2 weeks postpartum (or who have experienced a pregnancy loss) are considered to be at high risk for complications of influenza infection.1 Influenza A infection in pregnancy is associated with preterm labor, preterm birth, pneumonia, acute respiratory distress syndrome, and death.2 Although many pregnant patients may present with mild or moderate symptoms, the clinical progression with 2009 H1N1 appears to be more rapid than what has been seen with previous seasonal influenza outbreaks.3
According to 1 study, hospital admission rates during the first month of the outbreak were higher for pregnant women compared with the general population: 0.32 vs 0.076 per 100,000.4 The Centers for Disease Control and Prevention (CDC) indicates that while 1% of the population is pregnant at any given time, 6% of confirmed deaths from H1N1 in the United States in 2009 were pregnant women.5 Two prospective observational studies published in the Journal of the American Medical Association revealed the percentages of critically ill H1N1 patients who were pregnant. In Canada, 7.7% of critically ill patients with H1N1 were pregnant. In California, 10% were pregnant, and 6% of fatal cases in patients over age 18 were pregnant women.6,7
Why are pregnant women more susceptible to flu complications?
The immune system changes that make pregnant women more susceptible to complications of influenza infection are not well understood. Normal physiologic changes to the respiratory system during pregnancy may be a contributing factor. These include increased minute ventilation in the first trimester due to an increase in progesterone levels, increased tidal volume, decreased residual volume and functional residual capacity due to the mechanical effect of a gravid uterus, and increased oxygen consumption and basal metabolic rate due to increased demand.8
What can be done to decrease their risk?
The first step is preventing infection. For the 2009-2010 season, vaccination against seasonal and 2009 H1N1 influenza is strongly recommended for all pregnant women. Only the intramuscular injection is approved for pregnant women. Patients can receive the seasonal influenza vaccine at the same time as the H1N1 vaccine using an alternate injection site.
Maternal immunization against seasonal influenza benefits mothers and has also been shown to lower infection rates in infants. A study published in The New England Journal of Medicine showed that the seasonal influenza vaccination given to pregnant women reduced influenza-like illness in their infants younger than 6 months of age by 63%.9 Also important is providing chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection. For 2009 H1N1 chemoprophylaxis, a 10-day course of once-daily oseltamivir or zanamivir is acceptable. Zanamivir is an inhaled medication and should not be prescribed to patients with asthma or other respiratory conditions.
Confirmed case? Tx for the pregnant patient
The 2009 H1N1 virus is susceptible to oseltamivir and zanamivir.1 Both antivirals are Category C in pregnancy. The CDC recommends that patients with suspected or confirmed 2009 H1N1 infection who are in high-risk groups (which includes pregnant women) be treated with oseltamivir.
Antiviral medications such as oseltamivir and zanamivir act at the viral replication stage, which peaks at 24 to 72 hours in influenza.10 This helps explain evidence that the earlier treatment of influenza is initiated—within the first 48 hours—the more effective it is in reducing fever, relieving symptoms, and decreasing time to return to baseline activity.11 A study of pregnant women in California with severe 2009 H1N1 infection found that later treatment (>2 days after symptom onset) was associated with 4 times the risk of admission and death.3 For these reasons, treatment should not be delayed while test results are pending.
That said, in hospitalized patients with seasonal influenza, initiating treatment after 48 hours of symptom onset has been shown to provide some benefit in some observational studies.1,12 Consequently, the CDC recommends initiating treatment of high-risk patients who seek care more than 48 hours after symptom onset.1
The standard course of oseltamivir is 75 mg twice daily for 5 days. Longer courses may be beneficial in hospitalized patients.1 Oseltamivir and zanamivir can also be continued while breastfeeding.13
Our patient’s outcome
The patient received a 10-day course of oseltamivir (rather than the standard 5-day course), as well as empiric broad-spectrum antibiotic coverage for community-acquired pneumonia and aspiration pneumonia, including coverage for Streptococcus pneumoniae (the most common bacterial cause of secondary pneumonia in influenza14).
The cultures come back. Nasopharyngeal cultures were negative × 2 for type A influenza. Blood cultures were negative throughout the admission. Sputum cultures were negative, as well. Bronchoscopy cultures, however, were positive for type A influenza and negative for bacterial and fungal pathogens, confirming a diagnosis of primary pneumonia from 2009 H1N1 infection.
The patient was extubated 1 week after her arrival at our hospital and continued to recover during the rest of her hospital stay. She was discharged in stable condition. Several weeks later, she delivered a full-term infant with average weight and normal Apgar scores.
CORRESPONDENCE: Christopher Bernheisel, MD, Director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave., Suite 340, Cincinnati, OH 45219; [email protected]
- Don’t overestimate the value of a rapid influenza test. The sensitivity of these tests ranges between 10% and 70% in 2009 H1N1 influenza infection.
- Provide chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection.
- Consider longer courses of oseltamivir (beyond the standard 75 mg twice daily for 5 days) among hospitalized patients.
CASE: A 28-year-old woman (G6P1) at 33 weeks’ gestation was transferred from an outside hospital with worsening tachypnea, increasing oxygen requirement, and worsening infiltrates on chest radiograph.
A week earlier she had presented to a local emergency department (ED) with a 1-day history of nonproductive cough, fever, congestion, and decreased fetal movement. She also complained of vomiting. Examination was notable for an oxygen saturation of 99% on room air, heart rate of 126 bpm, temperature of 37.9°C (100.2°F), and blood pressure (BP) of 104/70 mm Hg. Rapid influenza A/B nasopharyngeal swab and group A Streptococcus direct probe were both negative. She was transferred to labor and delivery for fetal monitoring and discharged later that day.
Later in the week she returned to 2 other hospitals due to continued symptoms. She was diagnosed with right upper lobe pneumonia on her third ED visit and transferred to our facility, with increasing respiratory distress. Her examination was notable for a temperature of 36.8°C (98.2°F), pulse of 103 bpm, BP of 98/56 mm Hg, respiratory rate of 27 breaths per minute, and oxygen saturation of 94%. The patient had ulcerations on her tongue, dry mucous membranes, and lower extremity edema; on lung exam she had right lower lobe crackles and occasional wheezes.
Lab results were notable for a serum hemoglobin of 9.3 g/dL and platelet count of 75,000/mm3. The leukocyte count was 8.3×109/L, with differential remarkable for 24% bands. Potassium was 3.3 mEq/L and bicarbonate was 19 mEq/L; the basic metabolic panel was otherwise normal. Lactic acid was elevated at 2.4 mg/dL. Coagulation levels were normal. Urinalysis was negative. Chest radiograph (FIGURE) was read as “right upper lobe pneumonia and probable small bilateral pleural effusions with lower lung airspace disease, which may relate to atelectasis; however, superimposed multi-focal pneumonia is not excluded.”
Overnight, she had an increasing oxygen requirement of up to 15 liters, axillary temperature 40.8°C (105.6°F), and heart rate in the 140s; fetal heart rate was in the 200s. The next day, a chest x-ray revealed worsening pulmonary infiltrates. The patient was tachypneic, with a BP of 99/50 mm Hg. She continued to worsen and required intubation for hypoxic respiratory distress.
FIGURE
Right upper lobe pneumonia
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
H1N1 pneumonia
The patient’s physician initiated broad-spectrum antibiotics and oseltamivir for a presumptive diagnosis of 2009 H1N1 pneumonia and possible aspiration pneumonia. Although the patient had negative rapid influenza tests, the sensitivity of these tests is 10% to 70% in 2009 H1N1 influenza infection.1
Pregnant women and those in the first 2 weeks postpartum (or who have experienced a pregnancy loss) are considered to be at high risk for complications of influenza infection.1 Influenza A infection in pregnancy is associated with preterm labor, preterm birth, pneumonia, acute respiratory distress syndrome, and death.2 Although many pregnant patients may present with mild or moderate symptoms, the clinical progression with 2009 H1N1 appears to be more rapid than what has been seen with previous seasonal influenza outbreaks.3
According to 1 study, hospital admission rates during the first month of the outbreak were higher for pregnant women compared with the general population: 0.32 vs 0.076 per 100,000.4 The Centers for Disease Control and Prevention (CDC) indicates that while 1% of the population is pregnant at any given time, 6% of confirmed deaths from H1N1 in the United States in 2009 were pregnant women.5 Two prospective observational studies published in the Journal of the American Medical Association revealed the percentages of critically ill H1N1 patients who were pregnant. In Canada, 7.7% of critically ill patients with H1N1 were pregnant. In California, 10% were pregnant, and 6% of fatal cases in patients over age 18 were pregnant women.6,7
Why are pregnant women more susceptible to flu complications?
The immune system changes that make pregnant women more susceptible to complications of influenza infection are not well understood. Normal physiologic changes to the respiratory system during pregnancy may be a contributing factor. These include increased minute ventilation in the first trimester due to an increase in progesterone levels, increased tidal volume, decreased residual volume and functional residual capacity due to the mechanical effect of a gravid uterus, and increased oxygen consumption and basal metabolic rate due to increased demand.8
What can be done to decrease their risk?
The first step is preventing infection. For the 2009-2010 season, vaccination against seasonal and 2009 H1N1 influenza is strongly recommended for all pregnant women. Only the intramuscular injection is approved for pregnant women. Patients can receive the seasonal influenza vaccine at the same time as the H1N1 vaccine using an alternate injection site.
Maternal immunization against seasonal influenza benefits mothers and has also been shown to lower infection rates in infants. A study published in The New England Journal of Medicine showed that the seasonal influenza vaccination given to pregnant women reduced influenza-like illness in their infants younger than 6 months of age by 63%.9 Also important is providing chemoprophylaxis for pregnant women who have close contacts with suspected or confirmed influenza infection. For 2009 H1N1 chemoprophylaxis, a 10-day course of once-daily oseltamivir or zanamivir is acceptable. Zanamivir is an inhaled medication and should not be prescribed to patients with asthma or other respiratory conditions.
Confirmed case? Tx for the pregnant patient
The 2009 H1N1 virus is susceptible to oseltamivir and zanamivir.1 Both antivirals are Category C in pregnancy. The CDC recommends that patients with suspected or confirmed 2009 H1N1 infection who are in high-risk groups (which includes pregnant women) be treated with oseltamivir.
Antiviral medications such as oseltamivir and zanamivir act at the viral replication stage, which peaks at 24 to 72 hours in influenza.10 This helps explain evidence that the earlier treatment of influenza is initiated—within the first 48 hours—the more effective it is in reducing fever, relieving symptoms, and decreasing time to return to baseline activity.11 A study of pregnant women in California with severe 2009 H1N1 infection found that later treatment (>2 days after symptom onset) was associated with 4 times the risk of admission and death.3 For these reasons, treatment should not be delayed while test results are pending.
That said, in hospitalized patients with seasonal influenza, initiating treatment after 48 hours of symptom onset has been shown to provide some benefit in some observational studies.1,12 Consequently, the CDC recommends initiating treatment of high-risk patients who seek care more than 48 hours after symptom onset.1
The standard course of oseltamivir is 75 mg twice daily for 5 days. Longer courses may be beneficial in hospitalized patients.1 Oseltamivir and zanamivir can also be continued while breastfeeding.13
Our patient’s outcome
The patient received a 10-day course of oseltamivir (rather than the standard 5-day course), as well as empiric broad-spectrum antibiotic coverage for community-acquired pneumonia and aspiration pneumonia, including coverage for Streptococcus pneumoniae (the most common bacterial cause of secondary pneumonia in influenza14).
The cultures come back. Nasopharyngeal cultures were negative × 2 for type A influenza. Blood cultures were negative throughout the admission. Sputum cultures were negative, as well. Bronchoscopy cultures, however, were positive for type A influenza and negative for bacterial and fungal pathogens, confirming a diagnosis of primary pneumonia from 2009 H1N1 infection.
The patient was extubated 1 week after her arrival at our hospital and continued to recover during the rest of her hospital stay. She was discharged in stable condition. Several weeks later, she delivered a full-term infant with average weight and normal Apgar scores.
CORRESPONDENCE: Christopher Bernheisel, MD, Director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave., Suite 340, Cincinnati, OH 45219; [email protected]
1. Centers for Disease Control and Prevention. Updated interim recommendations for obstetric health care providers related to use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/pregnancy/antiviral_messages.htm. Accessed January 9, 2010.
2. Saleeby E, Chapman J, Morse J, et al. H1N1 Influenza in pregnancy: cause for concern. Obstet Gynecol. 2009;114:885-891.
3. Louie J, Acosta M, Jamieson D, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27-35.
4. Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
5. Centers for Disease Control and Prevention. 2009 H1N1 influenza vaccine and pregnant women: information for healthcare providers. Available at: http://www.cdc.gov/h1n1flu/vaccination/providers_qa.htm. Accessed January 9, 2010.
6. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872-1879.
7. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302:1896-1902.
8. Ratcliffe SD, Baxley EG, Cline MK, et al. Family Medicine Obstetrics. 3rd ed. Philadelphia, Pa: Mosby/Elsevier; 2008:203.
9. Zaman K, Roy E, Arifeen S, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555-1564.
10. Moscana A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363-1373.
11. Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123-129.
12. Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med. 2009;361:e110.-
13. United States National Library of Medicine. LactMed: drugs and lactation database. Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT. Accessed December 2, 2009.
14. Centers for Disease Control and Prevention. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325-329.
1. Centers for Disease Control and Prevention. Updated interim recommendations for obstetric health care providers related to use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/pregnancy/antiviral_messages.htm. Accessed January 9, 2010.
2. Saleeby E, Chapman J, Morse J, et al. H1N1 Influenza in pregnancy: cause for concern. Obstet Gynecol. 2009;114:885-891.
3. Louie J, Acosta M, Jamieson D, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27-35.
4. Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
5. Centers for Disease Control and Prevention. 2009 H1N1 influenza vaccine and pregnant women: information for healthcare providers. Available at: http://www.cdc.gov/h1n1flu/vaccination/providers_qa.htm. Accessed January 9, 2010.
6. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872-1879.
7. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302:1896-1902.
8. Ratcliffe SD, Baxley EG, Cline MK, et al. Family Medicine Obstetrics. 3rd ed. Philadelphia, Pa: Mosby/Elsevier; 2008:203.
9. Zaman K, Roy E, Arifeen S, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555-1564.
10. Moscana A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363-1373.
11. Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123-129.
12. Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med. 2009;361:e110.-
13. United States National Library of Medicine. LactMed: drugs and lactation database. Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT. Accessed December 2, 2009.
14. Centers for Disease Control and Prevention. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325-329.