User login
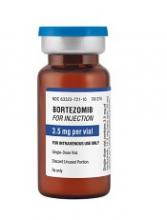
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()
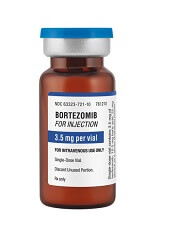
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()