User login
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
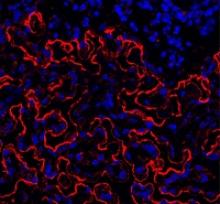
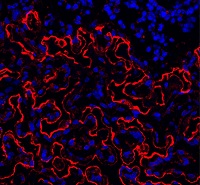
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()