User login
GlaxoSmithKline will be rolling out two-dimensional barcodes for most of its U.S.-licensed vaccines over the next several months, significantly increasing the number of vaccines carrying the high-tech labels.
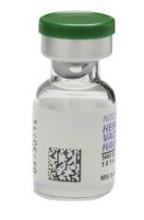
Current vaccine labels include linear, one-dimensional barcodes that contain the National Drug Code number, as required by the Food and Drug Administration. With the addition of seven vaccines from GSK later this year and early next year, however, the move to 2D barcoding is getting a big boost.
Previously, eight Sanofi Pasteur vaccines were available with the 2D barcodes, along with three vaccines from GSK. And more vaccine makers are preparing for the move to 2D barcodes.
"We’re committed and we’re on our way to have all of the U.S.-packaged vaccines with the 2D barcodes," said Dr. Leonard Friedland, vice president of scientific affairs and public policy for GSK Vaccines, North America. "In the future, we’ll have all of our vaccines [using 2D barcodes] as well, even those that are manufactured outside of the United States."
Today’s standard – linear barcodes – contain information on the National Drug Code number, but the lot number and expiration date of the vaccine are printed separately on the label. With the 2D data matrix technology, all of that information is contained within the barcode and can be transmitted electronically to either an electronic health record (EHR) system or a practice management system used to track inventory.
Supporters of the move to the 2D technology say that cutting down on the manual transcription of the lot number and expiration date has the potential to boost patient safety by improving the completeness of reports to the FDA’s Vaccine Adverse Events Reporting System (VAERS) or by making it easier to identify expired or recalled lots of vaccine.
It also has the potential to improve practice workflow, even in practices using paper records, by eliminating the need to record the information once into the paper chart and again into the state vaccine registry. The 2D barcoding allows practices to scan it directly into the registry. For practices with an EHR, there’s also the possibility of improved billing and just-in-time ordering, according to the American Academy of Pediatrics, which has been pushing vaccine manufacturers to adopt 2D barcodes for several years.
Dr. Edward Zissman, a pediatrician in Altamonte Springs, Fla., and cochair of the AAP’s Vaccine Barcoding Project, said that the primary motivator for pushing for the change in labeling was improving patient safety. A significant number of the primary reports to VAERS have an incorrect or unreadable lot number, he said.
If the manufacturer tried to recall a vaccine by lot number, it would be difficult to carry out at best, said Dr. Zissman.
Another driver in the move to 2D barcodes is the potential for making vaccine administration simpler for physicians and their staff. Entering the name, expiration date, lot number, and site of administration for multiple vaccines is time consuming and labor intensive, whether the office is working with paper records or an EHR. When there are shortages in a combination vaccine, as there are currently, and many children are getting a partial series of the vaccine, it becomes a "documentation nightmare," he said.
Whether the 2D barcodes will produce all the efficiency and cost savings that the AAP hopes is still an open question. Last year, the Centers for Disease Control and Prevention launched a pilot project to test how practices would do with the new barcodes and to determine best practices for tracking vaccines with the new technology. The early reports show that, by scanning 2D barcodes, practices are able to increase both the completeness and accuracy of the information they store and transmit on the vaccines. Practices also saw efficiency gains for inventory and administration.
But Dr. Zissman said the pilot’s results don’t tell the whole story because the study was limited to practices with an EHR, which may not reflect the experiences of the many paper-based offices across the United States, including Dr. Zissman’s own practice. The gains for paper-based practices will primarily be in the areas of inventory management and in the Immunization Information Systems (IIS), he said.
Although most vaccine manufacturers are now expediting implementation of the 2D barcodes, only two companies have rolled out the new labeling so far. It will now be up to the EHR vendors and the individual IISs to adopt 2D technology. Dr. Zissman said it’s his opinion that, where physicians have choices in purchasing vaccines they will buy from companies using the 2D barcodes. Unfortunately, he said, many vaccines are supplied by a single company, leaving physicians with no alternate choice.
Dr. Zissman has been a member of the Merck vaccine advisory board and is the principal investigator for pediatric clinical research studies for GSK, Merck, Novartis, and Medimmune.
On Twitter @MaryEllenNY
GlaxoSmithKline will be rolling out two-dimensional barcodes for most of its U.S.-licensed vaccines over the next several months, significantly increasing the number of vaccines carrying the high-tech labels.
Current vaccine labels include linear, one-dimensional barcodes that contain the National Drug Code number, as required by the Food and Drug Administration. With the addition of seven vaccines from GSK later this year and early next year, however, the move to 2D barcoding is getting a big boost.
Previously, eight Sanofi Pasteur vaccines were available with the 2D barcodes, along with three vaccines from GSK. And more vaccine makers are preparing for the move to 2D barcodes.
"We’re committed and we’re on our way to have all of the U.S.-packaged vaccines with the 2D barcodes," said Dr. Leonard Friedland, vice president of scientific affairs and public policy for GSK Vaccines, North America. "In the future, we’ll have all of our vaccines [using 2D barcodes] as well, even those that are manufactured outside of the United States."
Today’s standard – linear barcodes – contain information on the National Drug Code number, but the lot number and expiration date of the vaccine are printed separately on the label. With the 2D data matrix technology, all of that information is contained within the barcode and can be transmitted electronically to either an electronic health record (EHR) system or a practice management system used to track inventory.
Supporters of the move to the 2D technology say that cutting down on the manual transcription of the lot number and expiration date has the potential to boost patient safety by improving the completeness of reports to the FDA’s Vaccine Adverse Events Reporting System (VAERS) or by making it easier to identify expired or recalled lots of vaccine.
It also has the potential to improve practice workflow, even in practices using paper records, by eliminating the need to record the information once into the paper chart and again into the state vaccine registry. The 2D barcoding allows practices to scan it directly into the registry. For practices with an EHR, there’s also the possibility of improved billing and just-in-time ordering, according to the American Academy of Pediatrics, which has been pushing vaccine manufacturers to adopt 2D barcodes for several years.
Dr. Edward Zissman, a pediatrician in Altamonte Springs, Fla., and cochair of the AAP’s Vaccine Barcoding Project, said that the primary motivator for pushing for the change in labeling was improving patient safety. A significant number of the primary reports to VAERS have an incorrect or unreadable lot number, he said.
If the manufacturer tried to recall a vaccine by lot number, it would be difficult to carry out at best, said Dr. Zissman.
Another driver in the move to 2D barcodes is the potential for making vaccine administration simpler for physicians and their staff. Entering the name, expiration date, lot number, and site of administration for multiple vaccines is time consuming and labor intensive, whether the office is working with paper records or an EHR. When there are shortages in a combination vaccine, as there are currently, and many children are getting a partial series of the vaccine, it becomes a "documentation nightmare," he said.
Whether the 2D barcodes will produce all the efficiency and cost savings that the AAP hopes is still an open question. Last year, the Centers for Disease Control and Prevention launched a pilot project to test how practices would do with the new barcodes and to determine best practices for tracking vaccines with the new technology. The early reports show that, by scanning 2D barcodes, practices are able to increase both the completeness and accuracy of the information they store and transmit on the vaccines. Practices also saw efficiency gains for inventory and administration.
But Dr. Zissman said the pilot’s results don’t tell the whole story because the study was limited to practices with an EHR, which may not reflect the experiences of the many paper-based offices across the United States, including Dr. Zissman’s own practice. The gains for paper-based practices will primarily be in the areas of inventory management and in the Immunization Information Systems (IIS), he said.
Although most vaccine manufacturers are now expediting implementation of the 2D barcodes, only two companies have rolled out the new labeling so far. It will now be up to the EHR vendors and the individual IISs to adopt 2D technology. Dr. Zissman said it’s his opinion that, where physicians have choices in purchasing vaccines they will buy from companies using the 2D barcodes. Unfortunately, he said, many vaccines are supplied by a single company, leaving physicians with no alternate choice.
Dr. Zissman has been a member of the Merck vaccine advisory board and is the principal investigator for pediatric clinical research studies for GSK, Merck, Novartis, and Medimmune.
On Twitter @MaryEllenNY
GlaxoSmithKline will be rolling out two-dimensional barcodes for most of its U.S.-licensed vaccines over the next several months, significantly increasing the number of vaccines carrying the high-tech labels.
Current vaccine labels include linear, one-dimensional barcodes that contain the National Drug Code number, as required by the Food and Drug Administration. With the addition of seven vaccines from GSK later this year and early next year, however, the move to 2D barcoding is getting a big boost.
Previously, eight Sanofi Pasteur vaccines were available with the 2D barcodes, along with three vaccines from GSK. And more vaccine makers are preparing for the move to 2D barcodes.
"We’re committed and we’re on our way to have all of the U.S.-packaged vaccines with the 2D barcodes," said Dr. Leonard Friedland, vice president of scientific affairs and public policy for GSK Vaccines, North America. "In the future, we’ll have all of our vaccines [using 2D barcodes] as well, even those that are manufactured outside of the United States."
Today’s standard – linear barcodes – contain information on the National Drug Code number, but the lot number and expiration date of the vaccine are printed separately on the label. With the 2D data matrix technology, all of that information is contained within the barcode and can be transmitted electronically to either an electronic health record (EHR) system or a practice management system used to track inventory.
Supporters of the move to the 2D technology say that cutting down on the manual transcription of the lot number and expiration date has the potential to boost patient safety by improving the completeness of reports to the FDA’s Vaccine Adverse Events Reporting System (VAERS) or by making it easier to identify expired or recalled lots of vaccine.
It also has the potential to improve practice workflow, even in practices using paper records, by eliminating the need to record the information once into the paper chart and again into the state vaccine registry. The 2D barcoding allows practices to scan it directly into the registry. For practices with an EHR, there’s also the possibility of improved billing and just-in-time ordering, according to the American Academy of Pediatrics, which has been pushing vaccine manufacturers to adopt 2D barcodes for several years.
Dr. Edward Zissman, a pediatrician in Altamonte Springs, Fla., and cochair of the AAP’s Vaccine Barcoding Project, said that the primary motivator for pushing for the change in labeling was improving patient safety. A significant number of the primary reports to VAERS have an incorrect or unreadable lot number, he said.
If the manufacturer tried to recall a vaccine by lot number, it would be difficult to carry out at best, said Dr. Zissman.
Another driver in the move to 2D barcodes is the potential for making vaccine administration simpler for physicians and their staff. Entering the name, expiration date, lot number, and site of administration for multiple vaccines is time consuming and labor intensive, whether the office is working with paper records or an EHR. When there are shortages in a combination vaccine, as there are currently, and many children are getting a partial series of the vaccine, it becomes a "documentation nightmare," he said.
Whether the 2D barcodes will produce all the efficiency and cost savings that the AAP hopes is still an open question. Last year, the Centers for Disease Control and Prevention launched a pilot project to test how practices would do with the new barcodes and to determine best practices for tracking vaccines with the new technology. The early reports show that, by scanning 2D barcodes, practices are able to increase both the completeness and accuracy of the information they store and transmit on the vaccines. Practices also saw efficiency gains for inventory and administration.
But Dr. Zissman said the pilot’s results don’t tell the whole story because the study was limited to practices with an EHR, which may not reflect the experiences of the many paper-based offices across the United States, including Dr. Zissman’s own practice. The gains for paper-based practices will primarily be in the areas of inventory management and in the Immunization Information Systems (IIS), he said.
Although most vaccine manufacturers are now expediting implementation of the 2D barcodes, only two companies have rolled out the new labeling so far. It will now be up to the EHR vendors and the individual IISs to adopt 2D technology. Dr. Zissman said it’s his opinion that, where physicians have choices in purchasing vaccines they will buy from companies using the 2D barcodes. Unfortunately, he said, many vaccines are supplied by a single company, leaving physicians with no alternate choice.
Dr. Zissman has been a member of the Merck vaccine advisory board and is the principal investigator for pediatric clinical research studies for GSK, Merck, Novartis, and Medimmune.
On Twitter @MaryEllenNY