User login
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
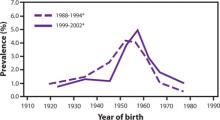
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
The Centers for Disease Control and Prevention (CDC) recently released new recommendations for screening for hepatitis C virus (HCV) infection that include a one-time screening for everyone in the United States born between 1945 and 1965, regardless of risk.1 These new recommendations are an enhancement of, but not a replacement for, the recommendations for HCV screening made in 1998, which called for screening those at high risk.2
HCV causes considerable morbidity and mortality in this country. Approximately 17,000 new infections occurred in 2010.1 Between 2.7 and 3.9 million Americans (1%-1.5% of the population) are living with chronic HCV infection, and many do not know they are infected.1 This lack of awareness appears to be due to a failure of both health care providers to offer testing to those at known risk and patients to either acknowledge or recall past high-risk behaviors.
Those at highest risk for HCV infection are current or past users of illegal injected drugs and recipients of a blood transfusion before 1992 (when HCV screening of the blood supply was instituted). Other risk factors are listed in TABLE 1.1 Many of those with HCV infection do not report injection drug use or having received a transfusion prior to 1992, and they are not detected by current risk-based testing.
TABLE 1
Risk factors for HCV infection1
| Most common risks |
|
| Less common risks |
|
| HCV, hepatitis C virus |
Approximately three-fourths of those who acquire HCV are unable to clear the virus and become chronically infected.1 Twenty percent of these individuals will develop cirrhosis and 5% will die from an HCV-related liver disease, such as decompensated cirrhosis or hepatocellular carcinoma (HCC).3
New treatments. In 2011, 2 protease inhibitor drugs, telaprevir and boceprevir, were approved for the treatment of HCV geno-type 1. These are the first generation of a class of drugs called direct-acting antiviral agents (DAAs). When a DAA is added to the standard therapy of ribavirin and pegylated interferon, the rate of viral clearance increases (from 44% to 75% for telaprevir and from 38% to 63% for boceprevir).1 However, the adverse reactions caused by these new drugs can lead to a 34% rise in the rate at which patients stop treatment.1 Twenty potential new HCV antivirals are in clinical trials, and it is expected that treatment recommendations will change rapidly as some of these are approved.
Does treatment improve long-term outcomes?
Clinical guidelines recommend antiviral treatment for anyone with HCV infection and biopsy evidence of bridging fibrosis, septal fibrosis, or cirrhosis.4
A look at Tx and all-cause mortality. Studies looking at patient-oriented outcomes such as all-cause mortality and incidence of HCC have been conducted with pegylated interferon and ribavirin, without the newer DAAs. The most commonly cited study assessed all-cause mortality in a large sample of veterans with multiple comorbidities.
Those who achieved a sustained virological response after treatment exhibited a reduction in all-cause mortality >50% compared with nonresponders. This endpoint included substantially lower rates of liver-related deaths and cirrhosis complicated by ascites, variceal bleeding, or encephalopathy.5 However, in such a nonrandom clinical trial, an improved outcome for responders could be due to their relatively good health, with fewer comorbid conditions, and other undetected biases. While this study attempted to control for such biases, it didn’t provide evidence that treating infection detected by screening a low-risk population would improve intermediate or long-term outcomes.
Observational studies look at carcinoma incidence. Twelve observational studies have addressed treatment effects on HCC incidence. They showed a 75% reduction in HCC rates in those who achieved viral clearance compared with those who did not.1 Again, these studies did not compare treated and untreated patients in a controlled clinical trial; they looked only at treated individuals and compared the outcomes of responders and nonresponders.
HCV transmission research is lacking. The CDC found no studies in its evidence review that addressed the issue of HCV transmission. Nevertheless, the new recommendations state that HCV transmission was a critical factor in determining the strength of the recommendation for age cohort screening. It is expected that those who have a sustained viral response will be less likely to transmit the virus to others.
Why the 1945-1965 birth cohort?
The prevalence of HCV infection in those born between 1945 and 1965 is 3.25%, and three-fourths of all those with HCV infection in the United States are in this cohort. The FIGURE depicts the large difference in prevalence between this age group and others. Within 3 cohorts defined by date of birth (1945-1965, 1950-1970, and 1945-1970), the prevalence of HCV infection is twice as high in men than in women, and in black non-Hispanics than in white non-Hispanics and Mexican Americans. However, extending the birth cohort to those born through 1970 yields only a marginal difference in prevalence figures. The CDC justifies restricting the new universal screening recommendation to the 1945-1965 age group mainly on the results of focus groups in which the public identified this cohort as “baby boomers” who would likely adopt the recommendation.
FIGURE
Prevalence of hepatitis C virus antibody by year of birth1
*National Health and Nutrition Examination Survey, United States, 1988–1994 and 1999–2002.
Two-step screening process
Screen individuals using a test for antibodies to HCV (anti-HCV). If the anti-HCV test result is positive, order a test for HCV nucleic acid that gives either a quantitative measure of viral load or a qualitative assessment of presence or absence of virus. If the confirmatory nucleic acid test result is negative, the individual does not have chronic HCV infection and is among the approximately 25% who clear the virus on their own. They do not need further testing or treatment.
What to tell infected patients
If the confirmatory test result is positive, presume the patient has HCV infection and offer the advice contained in TABLE 2.1 Patients should undergo further assessment for possible chronic liver disease and, with the counsel of their physician, decide whether to initiate treatment. They should also take measures to protect the liver from further damage, such as reducing alcohol consumption, avoiding medication and herbal products that can damage the liver, maintaining an optimal weight, and receiving vaccines against hepatitis A and B, if still susceptible to these viruses. Finally, encourage patients to take steps to avoid transmission of HCV to others.
TABLE 2
Advice for your patients with HCV infection1
Consult a health care provider (either a primary care physician or specialist [eg, in hepatology, gastroenterology, or infectious disease]) for:
|
Protect the liver from further harm by:
|
Maintain optimal weight by:
|
Minimize the risk of infecting others by:
|
| BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus. |
The decision on whether to begin treatment immediately is complicated by the large number of new antivirals in development, which will be available in the near future and may be more effective with fewer adverse effects.
Lingering controversies
Given the lack of evidence of improved outcomes with HCV screening in the general population, it will be interesting to see how widely accepted the new CDC recommendations will be. The US Preventive Services Task Force is in the process of revising its HCV screening recommendations. Given the Task Force’s evidence-based methodology and the lack of evidence on the benefits and harms of screening those with no reported risks, there may be some differences with the new CDC recommendations.
If the CDC’s assumption proves correct—ie, that the benefits of treating high-risk populations will also occur with treating detected infection in the general population—and if the age cohort screening recommendation is fully implemented, 47,000 cases of HCC and 15,000 liver transplants will be prevented.1
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
1. CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mortal Wkly Rep. 2012;61:1-18.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf. Accessed October 5, 2012.
2. CDC. Recommendations for prevention and control of hepatitis C virus infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1-54.
3. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
4. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
5. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virological response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.