User login
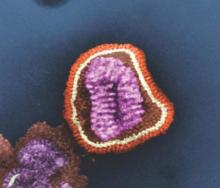
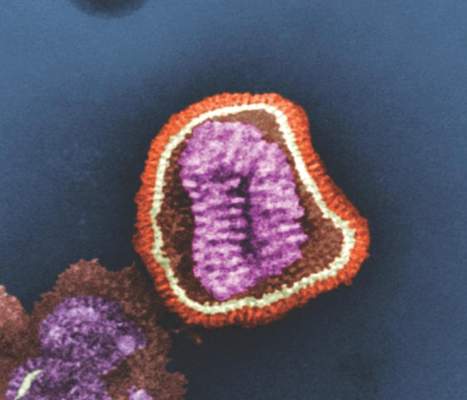
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.