User login
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
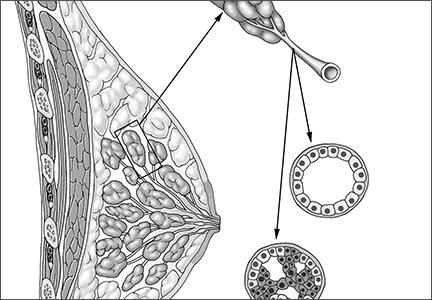
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A recent study reported in JAMA Oncology evaluated 10-year and 20-year breast cancer–specific mortality following diagnosis and treatment of ductal carcinoma in situ (DCIS) using the Surveillance, Epidemiology, and End Results (SEER) registries.1 The study included cases of pure DCIS (without lobular carcinoma in situ or microinvasion) diagnosed from 1988 to 2011 among women younger than age 70. It evaluated variables including age, race, income, type of surgery, radiation, subsequent diagnoses of invasive primary breast cancer, and, when applicable, cause of death.
Overall mortality rate was 3.3%
Mean follow-up was 7.5 years, with a 20-year breast cancer–specific mortality rate of 3.3% overall. Mortality was higher among young women diagnosed before the age of 35 years (7.8% vs 3.2%), and among black women (7.0% vs 3.0% for white women). The risk of dying from breast cancer was 18 times higher for women who developed subsequent ipsilateral invasive breast cancer. Mortality also was related to adverse DCIS characteristics such as grade, size, comedo-necrosis, and lack of an estrogen receptor.
Among patients who underwent lumpectomy, the addition of radiation reduced the risk of subsequent ipsilateral invasive breast cancer at 10 years (2.5% vs 4.9%; P<.001). However, radiation did not improve the 10-year rate of breast cancer mortality (0.8% for women who had lumpectomy with radiation, 0.9% for women who had lumpectomy alone, and 1.3% for women with unilateral mastectomy).
The prevention of ipsilateral invasive recurrence with radiation did not reduce mortality rates, as more than 50% of the women who died of breast cancer did not have an ipsilateral invasive recurrence prior to their death.
How these findings fit
into the larger picture
The findings of this landmark study confirm earlier reports, which showed that radiation after lumpectomy can reduce local recurrence but does not improve survival.2
Likewise, mastectomy, when compared with lumpectomy, offers no survival benefit and does not represent appropriate therapy for most women with small, unifocal DCIS.3
DCIS itself is not a life-threatening condition and has been described as a precursor lesion that, over 10 to 40 years, can lead to the development of invasive disease (FIGURE).4,5 High-grade DCIS tends to lead to high-grade invasive ductal carcinoma, and low-grade DCIS may develop into low-grade invasive disease.6
The increasing prevalence of screening mammography means that more small in situ lesions are being identified in US women. Unlike colonoscopy, which can prevent colon cancer by removing colon polyps, mammography with subsequent surgical treatment of DCIS has not reduced the incidence of invasive breast cancer.7This finding leads us to question whether all DCIS should be considered a precursor lesion. This well publicized study is generating controversy regarding overdiagnosis and overtreatment of DCIS.
Limitations of this study
The majority of patients in the SEER registry underwent surgical treatment of DCIS with or without radiation and had a survival rate of more than 97%. Because there was no untreated control group, this study does not allow us to draw any inferences on the role of expectant management of DCIS.
Although it is often declined by patients, tamoxifen reduces the risk of ipsilateral and contralateral invasive and in situ breast cancer. Regrettably, information on the use of adjuvant hormonal therapy after an initial diagnosis of DCIS was not included in this analysis.
Why did death from invasive
cancer sometimes follow a
diagnosis of DCIS?
Several factors could have contributed to the 3% mortality rate from invasive breast cancer among women in this large study of DCIS. For one, it is challenging for pathologists to perform comprehensive tissue sampling of mastectomy specimens—or even large lumpectomy specimens. Accordingly, occult microinvasive disease could be missed.8,9 As a result, occult invasive disease could go untreated, which could have contributed to the breast cancer mortality observed in this study.
Recommendations for practice
How can we better predict the behavior of DCIS and tailor treatment based on the biological behavior of each patient’s disease?
Individualize therapy. The likelihood of local invasive breast cancer recurrence should be estimated for each patient based on the size and grade of her disease. Furthermore, genetic profiling of DCIS has been developed with the Oncotype DX test (Genomic Health) multigene assay. This test can be performed on pathology specimens and has been shown to estimate the risk of in situ and invasive in-breast recurrence in patients who have undergone margin-negative lumpectomy for DCIS and who prefer to avoid radiation but are willing to take tamoxifen.10
Counsel precisely and accurately. Beyond such testing, we should focus on what is important to our patients in explaining the diagnosis:
- Our patients want to know that they are going to survive. Explain that DCIS is not a life-threatening cancer but a significant risk factor and is fully treatable with a long-term survival rate of 97%.
- Do not omit surgery. Follow-up surgical excision is still recommended after a core needle biopsy diagnosis of DCIS, as there is a 25% risk of finding invasive disease upon surgical excision.11,12 In our opinion, surgical excision represents the standard of care for DCIS, as some lesions may harbor invasive breast cancer.
- Explain the pros and cons of radiation to the patient once surgical excision has confirmed the diagnosis of pure DCIS. If the patient’s goal is to avoid any recurrence, then radiation can be useful and is particularly appropriate for women with high-grade, large, and estrogen-receptor–negative DCIS. However, patients in this setting need to recognize that radiation will not improve their already excellent rate of survival. For many patients, any recurrence, whether it’s DCIS or invasive disease, can be a devastating emotional event. But even in patients who experience a recurrence, early detection and treatment portend a very good outcome.
- Be aware of the fear of chemotherapy. Avoiding chemotherapy is a paramount (and understandable) desire for many women diagnosed with breast cancer. Women who choose radiation reduce their likelihood of invasive recurrence and potentially avoid the need for chemotherapy in the future.
- Know when mastectomy is indicated. Multicentric extensive DCIS is still an indication for mastectomy. The safety of avoiding mastectomy in this setting needs to be assessed by randomized trials. It may be safe for some women with DCIS, such as elderly patients with low-grade lesions, to undergo lumpectomy to rule out underlying invasive disease and be treated with endocrine therapy and observation, with or without radiation therapy. The issue of multiple re-excisions for close margins is also being re-evaluated.
Informed and shared
decision making is key
DCIS is an increasingly common and usually non–life-threatening condition. Radical surgery such as bilateral mastectomy for small unifocal DCIS is excessive and will not improve a patient’s outcome. As a prominent breast surgeon has written:
We must balance the small risk of breast cancer recurrence after lumpectomy for DCIS with patients’ quality of life concerns. This goal is best accomplished by using an informed and shared decision-making strategy to help our patients make sound decisions regarding DCIS.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
- Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896.
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- Page D, Rogers L, Schuyler P, et al. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, Recht A, Lagios M, eds. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:17–21.
- Sanders M, Schuyler P, Dupont W, Page D. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484.
- Burstein HJ, Plyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441.
- Lin C, Moore D, DeMichelle A, et al. The majority of locally advanced breast cancers are interval cancer [Abstract 1503]. J Clin Oncol. 2009;27.
- Lagios M, Westdahl P, Margolin F, Rose M. Duct carcinoma in situ: relationship of extend of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314.
- Schuh M, Nemoto T, Penetrante R, et al. Intraductal carcinoma: analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121(11):1303–1307.
- Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710.
- Bruening W, Schoelles K, Treadwell J, et al. Comparative effectiveness of core needle and open surgical biopsy for the diagnosis of breast lesions. Rockville, MD: Prepared by ECRI Institute for the Agency for Healthcare Research and Quality under contract No. 290-02-0019; Comparative Effectiveness Review; September 2008.
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128.
- Morrow M, Winograd JM, Freer PE, et al. Case records of the Massachusetts General Hospital. Case 8-2013: a 48-year-old woman with carcinoma in situ of the breast. N Engl J Med. 2013;368(11):1046–1053.
In this Article
- What is DCIS?
- Why did some deaths occur after diagnosis of DCIS?
- Why did some deaths occur after diagnosis of DCIS?