User login
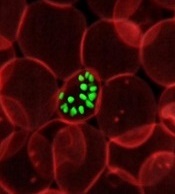
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()
Malaria parasites change the properties of red blood cells (RBCs) in a way that helps the parasites achieve cell entry, according to research published in PNAS.
Researchers found that Plasmodium parasites, upon binding to the surface of RBCs, cause the cell membranes to become more pliable, making it easier for the parasites to push inside the cells.
This suggests that differences in RBC stiffness, due to age or increased cholesterol content, could influence the parasites’ ability to invade.
“We have discovered that red cell entry is not just down to the ability of the parasite itself but that parasite-initiated changes to the red blood cells appear to contribute to the process of invasion,” said study author Marion Koch, a PhD student at Imperial College London in the UK.
“This could also mean that naturally more flexible cells would be easier for parasites to invade, which raises some interesting questions. Are parasites choosy about which cells to invade, picking the most deformable? Is susceptibility to malaria modified by fat or cholesterol content, or the age of circulating red blood cells?”
In the PNAS paper, the researchers noted that erythrocyte-binding antigen 175 (EBA175), a protein that’s required for entry in most parasite strains, binds to glycophorin A (GPA) on the RBC surface. However, the function of this binding interaction was unknown.
The team took a closer look at the interaction using real-time deformability cytometry and flicker spectroscopy.
They filmed 1000 RBCs per second passing through a narrow channel. Using this approach, the researchers were able to determine cell deformability by measuring how elongated the cells became during transit through the channel.
The team then measured where this deformation came from. They measured how much the RBCs deviate from their normally circular shape as their membranes naturally fluctuate or flicker.
The researchers found that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the RBC and a reduction in the bending modulus of the cell’s membrane. (The bending modulus is a measure of how much energy it takes to bend the cell membrane.)
The team then showed that the reduction in the bending modulus was “directly correlated with parasite invasion efficiency.”
The researchers said these results suggest the parasite primes the RBC surface through its binding antigens, altering the cell and reducing a barrier to invasion.
“This suggests we should be investigating not just parasite biology, but also how the body’s own red blood cells respond,” said study author Jake Baum, PhD, of Imperial College London.
“There are therapies developed for diseases like HIV that strengthen the body’s responses in addition to tackling the ‘invader.’ It’s not impossible to imagine something similar for malaria; for example, looking at a host-directed drug target and not just the parasite.” ![]()