User login
CASE: Erratic screening history with at least 1 abnormality
G.A., 40, vaguely remembers having at least 1 abnormal Pap test about 20 years ago. She is not sure exactly what the result was, but does recall that she underwent colposcopy. Her physician at the time told her that nothing important was detected and encouraged her to get “regular Pap tests” in the future. She followed this advice for several years, but her Pap tests became much less frequent after her 2 children were born. Today, she reports that her last Pap test was at least 5 years ago, but she does not remember exactly when. She also remembers being notified that she needed to repeat it, but is not sure why. Her records are unavailable because she has moved a lot and cannot remember the name of the doctor who saw her.
Today, G.A. is screened with both a Pap test and a human papillomavirus (HPV) test for high-risk viral types. The physician outlines the benefits of doing both tests and explains the “commonness” of HPV, offering reassuring facts about its natural history to “soften the concern” in the event she is found to be positive.
The Pap test comes back as “negative for intraepithelial neoplasia or malignancy” (ie, normal), but her HPV test is positive.
What questions is G.A. likely to raise? How can her risk of cervical cancer be quantified? And how should she be managed?
Why do an HPV test with the Pap?
G.A.’s situation is not unusual. Many women provide a vague history that includes 1 or more abnormal Pap tests in the distant past, with “probably normal” results on infrequent, irregular screening in more recent years.
It has been estimated that annual lifetime screening with a Pap test sensitivity of 70% for cervical intraepithelial neoplasia (CIN) 2 or 3 reduces the lifetime risk of cervical cancer by 93%. That means that even diligent lifetime screening will leave some women unprotected.1
The risk increases for women who are not screened regularly, especially those with a history of an abnormal Pap test. Approximately 10% of cervical cancers occur in women who have been screened in the past but not within the past 5 years.2
Pap test alone has poor sensitivity
A number of meta-analyses have documented the sensitivity of the conventional Pap smear for the detection of CIN 2,3 to be between 51% and 67%.3,4 And although liquid-based cytology offers many advantages, early reports of improved sensitivity over conventional cytology were not substantiated in a 2006 meta-analysis of a large number of studies (as reported in Update on Cervical Disease, by Thomas C. Wright, MD, in the March issue of OBG Management).5
Despite the low sensitivity of cervical cytology, Pap test screening has been extremely successful in detecting precancerous cervical changes and allowing timely treatment. The success is directly attributable to repeated screening of women during the relatively slow progression from initial HPV infection to CIN 3 (typically, about 10 years) and from CIN 3 to cancer (typically, 10 or more years).6 Poor sensitivity raises concern, however, when screening attendance is not ideal.
Together, the tests lower the risk of missing CIN or cancer to 1 in 1,000
Surely, G.A. would benefit by having the most reassuring screening available. Guidelines from the American Cancer Society (2002) and 2 practice bulletins from the American College of Obstetricians and Gynecologists (ACOG) (2003, 2005) recommend as 1 of 2 options screening women age 30 and over with both the Pap test and the HPV test for high-risk types. 7-9
A 2005 ACOG practice bulletin on HPV10 noted the reassurance offered by combined testing and observed that, based on Level A evidence, “HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, [so] women with concurrent negative test results can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000.”
Most women would benefit from a screening test that provides a reassurance of 1 in 1,000. Given G.A.’s infrequent screening history, previous abnormal cervical cytology, and unknown result on her last screen over 5 years ago, using the most reassuring combination of tests would seem to be imperative.
Likely questions
Because HPV testing plays an ever-increasing role in cervical screening, it presents a new set of educational challenges.6 Until recently, clinicians generally avoided discussing the cause of abnormal cervical cytology, CIN, and cervical cancer. However, when a woman is tested for high-risk HPV along with the Pap test in primary screening, the subject can no longer be skirted. Already the HPV vaccine and widespread use of the Web by patients have changed the information base on HPV. Education can begin at the time of the Pap and HPV tests, need not be extensive, and often can deflect undue anxiety and many of the patient’s questions about a positive result.
G.A. has been married for nearly 22 years. Her husband has been her only partner, but he was sexually active before they were married. She is naturally concerned about how long she has had HPV and what this means for her relationship. Her questions are universal: How and when did I become infected? What is my risk and that of my partner? How will I be managed? Will I always have HPV?
These questions may seem daunting, but the answers can be kept simple and short and still provide enough information to be reassuring and to prepare the patient for a possible positive test result.
Quantify risk before you select management
Most women with normal cytology but a first-time positive HPV test do not have CIN 2,3 or greater.11-14 A National Cancer Institute prospective 10-year follow-up of more than 20,000 women screened at enrollment with both the HPV test and cytology demonstrated that only 4.4% of the HPV-positive, Pap-negative women had CIN 3 or cancer detected over the following 3 to 5 years, and only 7% did by 10 years.13,14 These rates are similar to those found in other studies and are about half the risk represented by a Pap result of atypical squamous cells of undetermined significance (ASC-US).15
Therefore, because of the low immediate risk for high-grade cervical neoplasia and the extremely rare occurrence of cervical cancer in this setting, immediate referral to colposcopy is not recommended in routine cases (FIGURE 1).9,15 Instead, repeating Pap and HPV testing in 6 to 12 months yields a more accurate picture of risk by determining whether the HPV is only transient or is persistent. Only persistent HPV is associated with significant risk for CIN 2,3. Therefore, repeating the tests, rather than sending the patient to immediate colposcopy, allows the 45% to 70% of HPV infections destined to be transient to resolve spontaneously, but will still detect most significant lesions within a reasonable period of time.15
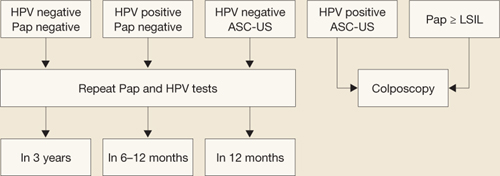
FIGURE 1 How to interpret the HPV test and cytology combined: NCI–ASCCP Interim Guidance
SOURCE: National Cancer Institute and the American Society for Colposcopy and Cervical Pathology. Adapted from Wright et al15
ASC-US=atypical squamous cells of undetermined significance; HPV=human papillomavirus; LSIL=low-grade squamous intraepithelial lesions.If G.A. is managed in this way and, at the 6- and 12-month repeats of both tests, has a positive HPV test (regardless of the cytology finding) or any abnormal Pap test result other than HPV-negative/ASC-US, she should undergo colposcopy.15 A test designated as HPV-negative/ASC-US can be managed by repeat testing in 12 months.
Remember: Even though a repeat positive HPV test increases the patient’s risk of CIN 2,3 significantly, it is specifically not recommended to treat the cervix solely on the basis of a persistently positive HPV test without evidence of CIN or cervical cancer.
This patient warrants a different approach—here’s why
Although repeat Pap and HPV testing in 6 to 12 months is the standard recommendation for women with a normal Pap test and positive HPV results, extenuating circumstances may exist. Clinical judgment always trumps routine recommendations in these cases.
The progression of CIN 3 to cervical cancer is usually a slow process that occurs over many years.6 Therefore, delaying colposcopy for 6 to 12 months will probably not increase risk significantly even if a high-grade lesion is already present. But G.A.’s case involves a number of variables that may increase her risk enough to justify immediate colposcopy:
- an abnormal Pap test more than 20 years ago
- a history of irregular screening
- no screening within the past 5 years until the current testing
- concern that her last Pap result was either minimally abnormal or of limited quality.
Lack of access to any earlier records further limits the physician’s ability to adequately judge G.A.’s risk. Because of these concerns, the physician asks G.A. to come in for colposcopy, at which time a 2-quadrant CIN 3 lesion is found (FIGURE 2). The patient is treated by loop electrosurgical excision procedure and has normal cytology and a negative HPV test result on follow-up.
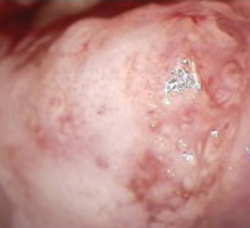
FIGURE 2 Two-quadrant CIN 2,3
High-grade lesions begin as a monoclonal cell change that enlarges centrifugally. Hence, increasing size is suspect for increasing duration of presence and increasing risk for cancer, because risk of invasion is proportional to lesion size.
Did you read Dr. Thomas C. Wright’s Update on Cervical Disease in the March Issue of OBG Management? If not, visit www.obgmanagement.com and follow the PAST ISSUES link on the navigation bar to the March issue.
The author reports no financial relationships relevant to this article.
1. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Br Med J (J Clin Res Ed). 1986;293:659-664.
2. Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283-2289.
3. Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810-819.
4. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
5. Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122-132.
6. Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 Suppl 3:S63-S70.
7. Saslow D, Runowicz CD, Solomon D, et al. For the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
8. Cervical cytology screening. American College of Obstetricians and Gynecologists Practice Bulletin #45. Washington, DC: ACOG; 2003.
9. Management of abnormal cervical cytology and histology. Clinical management guidelines for the Obstetrician and Gynecologist. American College of Obstetricians and Gynecologists Practice Bulletin #66. Washington, DC: ACOG; 2005.
10. Human papillomavirus. American College of Obstetricians and Gynecologists Practice Bulletin #61. Washington, DC: ACOG; 2005.
11. Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7,932 women. Br J Cancer. 2001;84:1616-1623.
12. Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871-1876.
13. Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46-52.
14. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
15. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
CASE: Erratic screening history with at least 1 abnormality
G.A., 40, vaguely remembers having at least 1 abnormal Pap test about 20 years ago. She is not sure exactly what the result was, but does recall that she underwent colposcopy. Her physician at the time told her that nothing important was detected and encouraged her to get “regular Pap tests” in the future. She followed this advice for several years, but her Pap tests became much less frequent after her 2 children were born. Today, she reports that her last Pap test was at least 5 years ago, but she does not remember exactly when. She also remembers being notified that she needed to repeat it, but is not sure why. Her records are unavailable because she has moved a lot and cannot remember the name of the doctor who saw her.
Today, G.A. is screened with both a Pap test and a human papillomavirus (HPV) test for high-risk viral types. The physician outlines the benefits of doing both tests and explains the “commonness” of HPV, offering reassuring facts about its natural history to “soften the concern” in the event she is found to be positive.
The Pap test comes back as “negative for intraepithelial neoplasia or malignancy” (ie, normal), but her HPV test is positive.
What questions is G.A. likely to raise? How can her risk of cervical cancer be quantified? And how should she be managed?
Why do an HPV test with the Pap?
G.A.’s situation is not unusual. Many women provide a vague history that includes 1 or more abnormal Pap tests in the distant past, with “probably normal” results on infrequent, irregular screening in more recent years.
It has been estimated that annual lifetime screening with a Pap test sensitivity of 70% for cervical intraepithelial neoplasia (CIN) 2 or 3 reduces the lifetime risk of cervical cancer by 93%. That means that even diligent lifetime screening will leave some women unprotected.1
The risk increases for women who are not screened regularly, especially those with a history of an abnormal Pap test. Approximately 10% of cervical cancers occur in women who have been screened in the past but not within the past 5 years.2
Pap test alone has poor sensitivity
A number of meta-analyses have documented the sensitivity of the conventional Pap smear for the detection of CIN 2,3 to be between 51% and 67%.3,4 And although liquid-based cytology offers many advantages, early reports of improved sensitivity over conventional cytology were not substantiated in a 2006 meta-analysis of a large number of studies (as reported in Update on Cervical Disease, by Thomas C. Wright, MD, in the March issue of OBG Management).5
Despite the low sensitivity of cervical cytology, Pap test screening has been extremely successful in detecting precancerous cervical changes and allowing timely treatment. The success is directly attributable to repeated screening of women during the relatively slow progression from initial HPV infection to CIN 3 (typically, about 10 years) and from CIN 3 to cancer (typically, 10 or more years).6 Poor sensitivity raises concern, however, when screening attendance is not ideal.
Together, the tests lower the risk of missing CIN or cancer to 1 in 1,000
Surely, G.A. would benefit by having the most reassuring screening available. Guidelines from the American Cancer Society (2002) and 2 practice bulletins from the American College of Obstetricians and Gynecologists (ACOG) (2003, 2005) recommend as 1 of 2 options screening women age 30 and over with both the Pap test and the HPV test for high-risk types. 7-9
A 2005 ACOG practice bulletin on HPV10 noted the reassurance offered by combined testing and observed that, based on Level A evidence, “HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, [so] women with concurrent negative test results can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000.”
Most women would benefit from a screening test that provides a reassurance of 1 in 1,000. Given G.A.’s infrequent screening history, previous abnormal cervical cytology, and unknown result on her last screen over 5 years ago, using the most reassuring combination of tests would seem to be imperative.
Likely questions
Because HPV testing plays an ever-increasing role in cervical screening, it presents a new set of educational challenges.6 Until recently, clinicians generally avoided discussing the cause of abnormal cervical cytology, CIN, and cervical cancer. However, when a woman is tested for high-risk HPV along with the Pap test in primary screening, the subject can no longer be skirted. Already the HPV vaccine and widespread use of the Web by patients have changed the information base on HPV. Education can begin at the time of the Pap and HPV tests, need not be extensive, and often can deflect undue anxiety and many of the patient’s questions about a positive result.
G.A. has been married for nearly 22 years. Her husband has been her only partner, but he was sexually active before they were married. She is naturally concerned about how long she has had HPV and what this means for her relationship. Her questions are universal: How and when did I become infected? What is my risk and that of my partner? How will I be managed? Will I always have HPV?
These questions may seem daunting, but the answers can be kept simple and short and still provide enough information to be reassuring and to prepare the patient for a possible positive test result.
Quantify risk before you select management
Most women with normal cytology but a first-time positive HPV test do not have CIN 2,3 or greater.11-14 A National Cancer Institute prospective 10-year follow-up of more than 20,000 women screened at enrollment with both the HPV test and cytology demonstrated that only 4.4% of the HPV-positive, Pap-negative women had CIN 3 or cancer detected over the following 3 to 5 years, and only 7% did by 10 years.13,14 These rates are similar to those found in other studies and are about half the risk represented by a Pap result of atypical squamous cells of undetermined significance (ASC-US).15
Therefore, because of the low immediate risk for high-grade cervical neoplasia and the extremely rare occurrence of cervical cancer in this setting, immediate referral to colposcopy is not recommended in routine cases (FIGURE 1).9,15 Instead, repeating Pap and HPV testing in 6 to 12 months yields a more accurate picture of risk by determining whether the HPV is only transient or is persistent. Only persistent HPV is associated with significant risk for CIN 2,3. Therefore, repeating the tests, rather than sending the patient to immediate colposcopy, allows the 45% to 70% of HPV infections destined to be transient to resolve spontaneously, but will still detect most significant lesions within a reasonable period of time.15

FIGURE 1 How to interpret the HPV test and cytology combined: NCI–ASCCP Interim Guidance
SOURCE: National Cancer Institute and the American Society for Colposcopy and Cervical Pathology. Adapted from Wright et al15
ASC-US=atypical squamous cells of undetermined significance; HPV=human papillomavirus; LSIL=low-grade squamous intraepithelial lesions.If G.A. is managed in this way and, at the 6- and 12-month repeats of both tests, has a positive HPV test (regardless of the cytology finding) or any abnormal Pap test result other than HPV-negative/ASC-US, she should undergo colposcopy.15 A test designated as HPV-negative/ASC-US can be managed by repeat testing in 12 months.
Remember: Even though a repeat positive HPV test increases the patient’s risk of CIN 2,3 significantly, it is specifically not recommended to treat the cervix solely on the basis of a persistently positive HPV test without evidence of CIN or cervical cancer.
This patient warrants a different approach—here’s why
Although repeat Pap and HPV testing in 6 to 12 months is the standard recommendation for women with a normal Pap test and positive HPV results, extenuating circumstances may exist. Clinical judgment always trumps routine recommendations in these cases.
The progression of CIN 3 to cervical cancer is usually a slow process that occurs over many years.6 Therefore, delaying colposcopy for 6 to 12 months will probably not increase risk significantly even if a high-grade lesion is already present. But G.A.’s case involves a number of variables that may increase her risk enough to justify immediate colposcopy:
- an abnormal Pap test more than 20 years ago
- a history of irregular screening
- no screening within the past 5 years until the current testing
- concern that her last Pap result was either minimally abnormal or of limited quality.
Lack of access to any earlier records further limits the physician’s ability to adequately judge G.A.’s risk. Because of these concerns, the physician asks G.A. to come in for colposcopy, at which time a 2-quadrant CIN 3 lesion is found (FIGURE 2). The patient is treated by loop electrosurgical excision procedure and has normal cytology and a negative HPV test result on follow-up.

FIGURE 2 Two-quadrant CIN 2,3
High-grade lesions begin as a monoclonal cell change that enlarges centrifugally. Hence, increasing size is suspect for increasing duration of presence and increasing risk for cancer, because risk of invasion is proportional to lesion size.
Did you read Dr. Thomas C. Wright’s Update on Cervical Disease in the March Issue of OBG Management? If not, visit www.obgmanagement.com and follow the PAST ISSUES link on the navigation bar to the March issue.
The author reports no financial relationships relevant to this article.
CASE: Erratic screening history with at least 1 abnormality
G.A., 40, vaguely remembers having at least 1 abnormal Pap test about 20 years ago. She is not sure exactly what the result was, but does recall that she underwent colposcopy. Her physician at the time told her that nothing important was detected and encouraged her to get “regular Pap tests” in the future. She followed this advice for several years, but her Pap tests became much less frequent after her 2 children were born. Today, she reports that her last Pap test was at least 5 years ago, but she does not remember exactly when. She also remembers being notified that she needed to repeat it, but is not sure why. Her records are unavailable because she has moved a lot and cannot remember the name of the doctor who saw her.
Today, G.A. is screened with both a Pap test and a human papillomavirus (HPV) test for high-risk viral types. The physician outlines the benefits of doing both tests and explains the “commonness” of HPV, offering reassuring facts about its natural history to “soften the concern” in the event she is found to be positive.
The Pap test comes back as “negative for intraepithelial neoplasia or malignancy” (ie, normal), but her HPV test is positive.
What questions is G.A. likely to raise? How can her risk of cervical cancer be quantified? And how should she be managed?
Why do an HPV test with the Pap?
G.A.’s situation is not unusual. Many women provide a vague history that includes 1 or more abnormal Pap tests in the distant past, with “probably normal” results on infrequent, irregular screening in more recent years.
It has been estimated that annual lifetime screening with a Pap test sensitivity of 70% for cervical intraepithelial neoplasia (CIN) 2 or 3 reduces the lifetime risk of cervical cancer by 93%. That means that even diligent lifetime screening will leave some women unprotected.1
The risk increases for women who are not screened regularly, especially those with a history of an abnormal Pap test. Approximately 10% of cervical cancers occur in women who have been screened in the past but not within the past 5 years.2
Pap test alone has poor sensitivity
A number of meta-analyses have documented the sensitivity of the conventional Pap smear for the detection of CIN 2,3 to be between 51% and 67%.3,4 And although liquid-based cytology offers many advantages, early reports of improved sensitivity over conventional cytology were not substantiated in a 2006 meta-analysis of a large number of studies (as reported in Update on Cervical Disease, by Thomas C. Wright, MD, in the March issue of OBG Management).5
Despite the low sensitivity of cervical cytology, Pap test screening has been extremely successful in detecting precancerous cervical changes and allowing timely treatment. The success is directly attributable to repeated screening of women during the relatively slow progression from initial HPV infection to CIN 3 (typically, about 10 years) and from CIN 3 to cancer (typically, 10 or more years).6 Poor sensitivity raises concern, however, when screening attendance is not ideal.
Together, the tests lower the risk of missing CIN or cancer to 1 in 1,000
Surely, G.A. would benefit by having the most reassuring screening available. Guidelines from the American Cancer Society (2002) and 2 practice bulletins from the American College of Obstetricians and Gynecologists (ACOG) (2003, 2005) recommend as 1 of 2 options screening women age 30 and over with both the Pap test and the HPV test for high-risk types. 7-9
A 2005 ACOG practice bulletin on HPV10 noted the reassurance offered by combined testing and observed that, based on Level A evidence, “HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, [so] women with concurrent negative test results can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000.”
Most women would benefit from a screening test that provides a reassurance of 1 in 1,000. Given G.A.’s infrequent screening history, previous abnormal cervical cytology, and unknown result on her last screen over 5 years ago, using the most reassuring combination of tests would seem to be imperative.
Likely questions
Because HPV testing plays an ever-increasing role in cervical screening, it presents a new set of educational challenges.6 Until recently, clinicians generally avoided discussing the cause of abnormal cervical cytology, CIN, and cervical cancer. However, when a woman is tested for high-risk HPV along with the Pap test in primary screening, the subject can no longer be skirted. Already the HPV vaccine and widespread use of the Web by patients have changed the information base on HPV. Education can begin at the time of the Pap and HPV tests, need not be extensive, and often can deflect undue anxiety and many of the patient’s questions about a positive result.
G.A. has been married for nearly 22 years. Her husband has been her only partner, but he was sexually active before they were married. She is naturally concerned about how long she has had HPV and what this means for her relationship. Her questions are universal: How and when did I become infected? What is my risk and that of my partner? How will I be managed? Will I always have HPV?
These questions may seem daunting, but the answers can be kept simple and short and still provide enough information to be reassuring and to prepare the patient for a possible positive test result.
Quantify risk before you select management
Most women with normal cytology but a first-time positive HPV test do not have CIN 2,3 or greater.11-14 A National Cancer Institute prospective 10-year follow-up of more than 20,000 women screened at enrollment with both the HPV test and cytology demonstrated that only 4.4% of the HPV-positive, Pap-negative women had CIN 3 or cancer detected over the following 3 to 5 years, and only 7% did by 10 years.13,14 These rates are similar to those found in other studies and are about half the risk represented by a Pap result of atypical squamous cells of undetermined significance (ASC-US).15
Therefore, because of the low immediate risk for high-grade cervical neoplasia and the extremely rare occurrence of cervical cancer in this setting, immediate referral to colposcopy is not recommended in routine cases (FIGURE 1).9,15 Instead, repeating Pap and HPV testing in 6 to 12 months yields a more accurate picture of risk by determining whether the HPV is only transient or is persistent. Only persistent HPV is associated with significant risk for CIN 2,3. Therefore, repeating the tests, rather than sending the patient to immediate colposcopy, allows the 45% to 70% of HPV infections destined to be transient to resolve spontaneously, but will still detect most significant lesions within a reasonable period of time.15

FIGURE 1 How to interpret the HPV test and cytology combined: NCI–ASCCP Interim Guidance
SOURCE: National Cancer Institute and the American Society for Colposcopy and Cervical Pathology. Adapted from Wright et al15
ASC-US=atypical squamous cells of undetermined significance; HPV=human papillomavirus; LSIL=low-grade squamous intraepithelial lesions.If G.A. is managed in this way and, at the 6- and 12-month repeats of both tests, has a positive HPV test (regardless of the cytology finding) or any abnormal Pap test result other than HPV-negative/ASC-US, she should undergo colposcopy.15 A test designated as HPV-negative/ASC-US can be managed by repeat testing in 12 months.
Remember: Even though a repeat positive HPV test increases the patient’s risk of CIN 2,3 significantly, it is specifically not recommended to treat the cervix solely on the basis of a persistently positive HPV test without evidence of CIN or cervical cancer.
This patient warrants a different approach—here’s why
Although repeat Pap and HPV testing in 6 to 12 months is the standard recommendation for women with a normal Pap test and positive HPV results, extenuating circumstances may exist. Clinical judgment always trumps routine recommendations in these cases.
The progression of CIN 3 to cervical cancer is usually a slow process that occurs over many years.6 Therefore, delaying colposcopy for 6 to 12 months will probably not increase risk significantly even if a high-grade lesion is already present. But G.A.’s case involves a number of variables that may increase her risk enough to justify immediate colposcopy:
- an abnormal Pap test more than 20 years ago
- a history of irregular screening
- no screening within the past 5 years until the current testing
- concern that her last Pap result was either minimally abnormal or of limited quality.
Lack of access to any earlier records further limits the physician’s ability to adequately judge G.A.’s risk. Because of these concerns, the physician asks G.A. to come in for colposcopy, at which time a 2-quadrant CIN 3 lesion is found (FIGURE 2). The patient is treated by loop electrosurgical excision procedure and has normal cytology and a negative HPV test result on follow-up.

FIGURE 2 Two-quadrant CIN 2,3
High-grade lesions begin as a monoclonal cell change that enlarges centrifugally. Hence, increasing size is suspect for increasing duration of presence and increasing risk for cancer, because risk of invasion is proportional to lesion size.
Did you read Dr. Thomas C. Wright’s Update on Cervical Disease in the March Issue of OBG Management? If not, visit www.obgmanagement.com and follow the PAST ISSUES link on the navigation bar to the March issue.
The author reports no financial relationships relevant to this article.
1. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Br Med J (J Clin Res Ed). 1986;293:659-664.
2. Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283-2289.
3. Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810-819.
4. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
5. Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122-132.
6. Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 Suppl 3:S63-S70.
7. Saslow D, Runowicz CD, Solomon D, et al. For the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
8. Cervical cytology screening. American College of Obstetricians and Gynecologists Practice Bulletin #45. Washington, DC: ACOG; 2003.
9. Management of abnormal cervical cytology and histology. Clinical management guidelines for the Obstetrician and Gynecologist. American College of Obstetricians and Gynecologists Practice Bulletin #66. Washington, DC: ACOG; 2005.
10. Human papillomavirus. American College of Obstetricians and Gynecologists Practice Bulletin #61. Washington, DC: ACOG; 2005.
11. Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7,932 women. Br J Cancer. 2001;84:1616-1623.
12. Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871-1876.
13. Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46-52.
14. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
15. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
1. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Br Med J (J Clin Res Ed). 1986;293:659-664.
2. Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283-2289.
3. Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810-819.
4. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680-689.
5. Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122-132.
6. Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 Suppl 3:S63-S70.
7. Saslow D, Runowicz CD, Solomon D, et al. For the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
8. Cervical cytology screening. American College of Obstetricians and Gynecologists Practice Bulletin #45. Washington, DC: ACOG; 2003.
9. Management of abnormal cervical cytology and histology. Clinical management guidelines for the Obstetrician and Gynecologist. American College of Obstetricians and Gynecologists Practice Bulletin #66. Washington, DC: ACOG; 2005.
10. Human papillomavirus. American College of Obstetricians and Gynecologists Practice Bulletin #61. Washington, DC: ACOG; 2005.
11. Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7,932 women. Br J Cancer. 2001;84:1616-1623.
12. Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871-1876.
13. Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46-52.
14. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
15. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
IN THIS ARTICLE