User login
Clinicians can do more to improve human papillomavirus vaccination coverage rates among adolescent females, including emphasizing to parents that the vaccine prevents cancer, according to Dr. Thomas Frieden, the director of the Centers for Disease Control and Prevention.
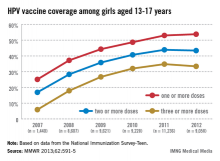
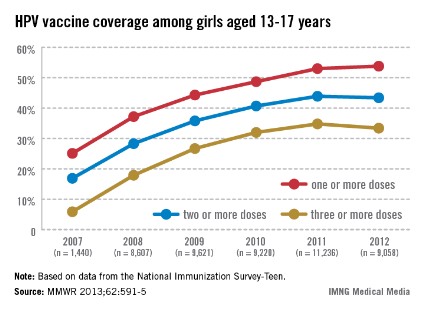
In 2011, only 35% of girls aged 13-17 years had received the full three doses of the HPV vaccine, which dropped slightly in 2012, which is "a huge disappointment," Dr. Frieden said during a July 25 telebriefing. The proportion of adolescent girls who received at least one, two, and the full three-dose HPV vaccine series increased every year from 2007 to 2011, but stalled between 2011 and 2012 and remains at unacceptably low levels – while missed opportunities for vaccination in this population have increased markedly, according to the data (MMWR 2013;62:591-5).
But rates can be increased if clinicians take every possible opportunity to vaccinate adolescents with the HPV vaccine, including when they receive other vaccines, he said during the briefing. While administering a three-dose series was thought to be difficult and was behind the low rates, the data indicated that if the HPV vaccine was given every time the adolescents received another vaccine, the completion of the three-dose series could increase to 93%, he said. According to the report, "Every health care visit, whether for back-to-school evaluations or acute problems, should be used to assess teenagers’ immunization status and provide recommended vaccines if indicated."
"If we get the three-dose series to 80%, an estimated 53,000 cases of cervical cancer could be prevented over the lifetimes of girls aged 12 [years] and younger," Dr. Frieden said.
A three-dose series of Gardasil – the quadrivalent HPV vaccine directed against HPV-16 and -18 (which cause most cervical cancers) and HPV-6 and -11 (which cause most genital warts) – was licensed and recommended by the CDC as a routine vaccine in 2006 for females aged 11-12 years and for females aged 13-26 years who had not been vaccinated. In 2009, the bivalent vaccine Cervarix, directed against HPV-16 and -18, was licensed for use in females aged 10-25 years and added to the recommendation in 2009.
The American Academy of Pediatrics "shares CDC’s disappointment and concern that we are not providing this lifesaving vaccine at the same rates as we do with other immunizations," Dr. Thomas K. McInerny, president of the AAP, said during the briefing.
"Use every opportunity to vaccinate your adolescent patients at both illness and well-child visits," as well as visits for sports physicals, he advised. He also recommended using alerts in electronic medical record systems so vaccination status is reviewed at every patient visit, as well as reminding parents with automated postcards or phone calls, having nurses check vaccine status when they bring patients into the exam room, and implementing standing order policies so that patients can receive vaccines without a physician’s exam or a physician’s order.
The vaccination coverage data were from the National Immunization Survey–Teen (NIS-Teen), which collects data on vaccination among adolescents aged 13-17 years in the 50 states, the District of Columbia, and certain areas of the country, including New York City, and Chicago. Data on HPV vaccination rates among adolescent males will be reported in the future.
In 2007, 25.1% of adolescent girls aged 13-17 years had received at least one dose of the HPV vaccine, which increased to 53% in 2011 and to only 53.8% in 2012. The proportion of adolescent girls who had received the full three-dose series was even lower, increasing from 5.9% in 2007 to 34.8% in 2011 and dropping slightly in 2012 to 33.4%.
The report also provides estimates of missed opportunities for vaccinating adolescent girls, defined as "a health-care encounter occurring on or after a girl’s 11th birthday," and on or after March 23, 2007. Missed opportunities increased from 20.8% in 2007 to 84% in 2012. (March 23, 2007, was when the CDC’s Advisory Committee on Immunization Practices (ACIP) recommendation on the quadrivalent vaccine was published).
The survey found that 23% of parents said they did not plan to get their daughters vaccinated in the next year. The most common reason cited by these parents is that they did not believe the vaccine was necessary (19.1%), followed by concerns about safety (13.1%), vaccine not recommended (14.2%), lack of knowledge of the vaccine or the disease (12.6%), and their daughter was not sexually active (10.1%). Cost did not appear to be an issue.
The report also included safety data, based on the estimated 56 million doses of the quadrivalent vaccine distributed in the United States from June 2006 through March 2013, which did not identify any new safety issues associated with the HPV vaccine, Dr. Frieden said. (Safety focused on the quadrivalent vaccine because most of the vaccine used has been this vaccine).
Most – 92.1% – of the 21,194 adverse event reports received by the Vaccine Adverse Event Reporting System (VAERS) were classified as "nonserious," and included syncope, dizziness, nausea, headache, fever, and urticaria. Local effects included injection-site pain, redness and swelling. Only 8% of the reports were classified as serious and included headache, vomiting, fatigue, dizziness, syncope, and generalized weakness.
The report also points out that no serious safety concerns have been identified in three population-based U.S. studies of the quadrivalent vaccine safety, although one observational study since the vaccine was licensed found an increased syncope risk.
Dr. Frieden noted that an increased risk of syncope may not be specific to the HPV vaccine because syncope is associated with other vaccines among adolescents, and that a 15-minute observation period after the vaccine is administered is recommended.
He referred to a report released in June in the Journal of Infectious Diseases, which found that the infections with the HPV types in the quadrivalent HPV vaccine dropped by almost 60% among females aged 14-19 years during the 4-year period after the vaccine became available and was recommended as a routine vaccine. (J. Inf. Dis. 2013; 208:385-93).
In the United States every year, an estimated 14 million people will become infected with HPV and about 26,200 will develop new cancers from HPV: 17,400 of those cancers are among females, 10,300 of which are cervical cancers, and 8,800 of the cancers are among males, of which 6,700 are oropharyngeal cancers.
In 2011, the recommendation for the quadrivalent vaccine was expanded to include boys aged 11-12 years, and for unvaccinated males up to age 26 years.
For those senior pediatricians such as me, the importance of vaccines to
child and adolescent health such as Hemophilus influenzae, pneumococcal
vaccine and others is a "no brainer." We recall the devastating
diseases that are no longer commonplace, because of prevention by
immunization. In the United States, 14 million people will become
infected with HPV every year and 26,200 will develop new cancers from
HPV, almost half of these cervical cancer. As Dr. Frieden pointed out in
the telebriefing, even the small percentage of girls who have received
with the HPV vaccine has made a difference, reducing HPV infections by
almost 60% among females aged 14-19 years in just 4 years.
The HPV vaccine series has met with an initial difficult reception from parents, patients, and providers. Although, at first, the cost of approximately $380 for the three-shot series was a barrier, over time this has become less of an obstacle, and interestingly, this issue was not reported to be a barrier to receiving the vaccine series in the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices survey on the quadrivalent series. Current lassitude has resulted in only 33.4% of 13- to 17-year-olds being fully immunized in 2012, as reported in the MMWR.
In the same survey, missed opportunities to provide this vaccine after a girl's 11th birthday increased from 20.8% to 84%. Clearly, recommendations to refine systems of delivery in physicians' offices and the use of electronic health records (EHR) to prime providers to immunize at every opportunity in the clinical setting are good ideas. But an undercurrent that is often not openly discussed is that physicians often don't ask or wish to "take on" negative parental reaction to this important vaccine that can prevent cancer but is often linked to reproductive behavior. The link with acquisition of this virus through sexual activity is one that parents often don't want to consider, and frequently the provider acquiesces. In the ACIP survey, the most common reason cited by parents is that they do not believe the vaccine was necessary, followed by concerns of safety, that the vaccine was not recommended, lack of knowledge about the disease, and, finally, that their daughter was not sexually active.
Pediatricians must use every visit to review immunization status. When in an exam room, HPV should be included in the vaccines that are given at a health visit to prevent meningitis and pertussis. For example, say, "Maggie is due to receive these shots today; they are HPV, Menactra, and Tdap."* Too often, other physicians and I timidly approach the HPV vaccine series and allow parents' lack of knowledge - or worse, media hearsay - to dictate a youngster's care and, they opt to not immunize. Moreover, we often don't even have the conversation!
In the clinical trenches, if a parent questions why her daughter or son needs the vaccine, a directed but not overly lengthy response of the importance of this vaccine in preventing cervical and other cancers will inform the parent and teen, and is generally well received. Often, asking if an older family member or friend has suffered with cervical cancer will allow the parent to acknowledge that this is a reality that they do not want their teen to experience. When we fail to recommend the HPV series or say its fine "to wait" because of an inability and unwillingness to raise the link of acquisition of HPV and sexual activity, an opportunity is missed.
The media has done much to promote positive health behaviors, but sadly, parents often recall only the negatives associated with the HPV vaccine. Both clinicians and parents must repurpose themselves to protecting children and adolescents. Parents and youth rely on their pediatrician's recommendations and knowledge. In brief, pediatricians must pin a Post It note to their EHR to cue them to educate, but then vaccinate! In short, "Do Ask, Do Tell." No opportunity to protect our youth should be lost!
Dr. Susan Jay is program director of adolescent health and medicine at Children's Hospital of Wisconsin in Wauwatosa, and a professor of pediatrics at the Medical College of Wisconsin. Dr. Jay has no relevant financial disclosures.
*Correction 8/5/2013: The vaccine was misstated as DTap; teens receive Tdap.
This story was updated. 8/5/2013
For those senior pediatricians such as me, the importance of vaccines to
child and adolescent health such as Hemophilus influenzae, pneumococcal
vaccine and others is a "no brainer." We recall the devastating
diseases that are no longer commonplace, because of prevention by
immunization. In the United States, 14 million people will become
infected with HPV every year and 26,200 will develop new cancers from
HPV, almost half of these cervical cancer. As Dr. Frieden pointed out in
the telebriefing, even the small percentage of girls who have received
with the HPV vaccine has made a difference, reducing HPV infections by
almost 60% among females aged 14-19 years in just 4 years.
The HPV vaccine series has met with an initial difficult reception from parents, patients, and providers. Although, at first, the cost of approximately $380 for the three-shot series was a barrier, over time this has become less of an obstacle, and interestingly, this issue was not reported to be a barrier to receiving the vaccine series in the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices survey on the quadrivalent series. Current lassitude has resulted in only 33.4% of 13- to 17-year-olds being fully immunized in 2012, as reported in the MMWR.
In the same survey, missed opportunities to provide this vaccine after a girl's 11th birthday increased from 20.8% to 84%. Clearly, recommendations to refine systems of delivery in physicians' offices and the use of electronic health records (EHR) to prime providers to immunize at every opportunity in the clinical setting are good ideas. But an undercurrent that is often not openly discussed is that physicians often don't ask or wish to "take on" negative parental reaction to this important vaccine that can prevent cancer but is often linked to reproductive behavior. The link with acquisition of this virus through sexual activity is one that parents often don't want to consider, and frequently the provider acquiesces. In the ACIP survey, the most common reason cited by parents is that they do not believe the vaccine was necessary, followed by concerns of safety, that the vaccine was not recommended, lack of knowledge about the disease, and, finally, that their daughter was not sexually active.
Pediatricians must use every visit to review immunization status. When in an exam room, HPV should be included in the vaccines that are given at a health visit to prevent meningitis and pertussis. For example, say, "Maggie is due to receive these shots today; they are HPV, Menactra, and Tdap."* Too often, other physicians and I timidly approach the HPV vaccine series and allow parents' lack of knowledge - or worse, media hearsay - to dictate a youngster's care and, they opt to not immunize. Moreover, we often don't even have the conversation!
In the clinical trenches, if a parent questions why her daughter or son needs the vaccine, a directed but not overly lengthy response of the importance of this vaccine in preventing cervical and other cancers will inform the parent and teen, and is generally well received. Often, asking if an older family member or friend has suffered with cervical cancer will allow the parent to acknowledge that this is a reality that they do not want their teen to experience. When we fail to recommend the HPV series or say its fine "to wait" because of an inability and unwillingness to raise the link of acquisition of HPV and sexual activity, an opportunity is missed.
The media has done much to promote positive health behaviors, but sadly, parents often recall only the negatives associated with the HPV vaccine. Both clinicians and parents must repurpose themselves to protecting children and adolescents. Parents and youth rely on their pediatrician's recommendations and knowledge. In brief, pediatricians must pin a Post It note to their EHR to cue them to educate, but then vaccinate! In short, "Do Ask, Do Tell." No opportunity to protect our youth should be lost!
Dr. Susan Jay is program director of adolescent health and medicine at Children's Hospital of Wisconsin in Wauwatosa, and a professor of pediatrics at the Medical College of Wisconsin. Dr. Jay has no relevant financial disclosures.
*Correction 8/5/2013: The vaccine was misstated as DTap; teens receive Tdap.
This story was updated. 8/5/2013
For those senior pediatricians such as me, the importance of vaccines to
child and adolescent health such as Hemophilus influenzae, pneumococcal
vaccine and others is a "no brainer." We recall the devastating
diseases that are no longer commonplace, because of prevention by
immunization. In the United States, 14 million people will become
infected with HPV every year and 26,200 will develop new cancers from
HPV, almost half of these cervical cancer. As Dr. Frieden pointed out in
the telebriefing, even the small percentage of girls who have received
with the HPV vaccine has made a difference, reducing HPV infections by
almost 60% among females aged 14-19 years in just 4 years.
The HPV vaccine series has met with an initial difficult reception from parents, patients, and providers. Although, at first, the cost of approximately $380 for the three-shot series was a barrier, over time this has become less of an obstacle, and interestingly, this issue was not reported to be a barrier to receiving the vaccine series in the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices survey on the quadrivalent series. Current lassitude has resulted in only 33.4% of 13- to 17-year-olds being fully immunized in 2012, as reported in the MMWR.
In the same survey, missed opportunities to provide this vaccine after a girl's 11th birthday increased from 20.8% to 84%. Clearly, recommendations to refine systems of delivery in physicians' offices and the use of electronic health records (EHR) to prime providers to immunize at every opportunity in the clinical setting are good ideas. But an undercurrent that is often not openly discussed is that physicians often don't ask or wish to "take on" negative parental reaction to this important vaccine that can prevent cancer but is often linked to reproductive behavior. The link with acquisition of this virus through sexual activity is one that parents often don't want to consider, and frequently the provider acquiesces. In the ACIP survey, the most common reason cited by parents is that they do not believe the vaccine was necessary, followed by concerns of safety, that the vaccine was not recommended, lack of knowledge about the disease, and, finally, that their daughter was not sexually active.
Pediatricians must use every visit to review immunization status. When in an exam room, HPV should be included in the vaccines that are given at a health visit to prevent meningitis and pertussis. For example, say, "Maggie is due to receive these shots today; they are HPV, Menactra, and Tdap."* Too often, other physicians and I timidly approach the HPV vaccine series and allow parents' lack of knowledge - or worse, media hearsay - to dictate a youngster's care and, they opt to not immunize. Moreover, we often don't even have the conversation!
In the clinical trenches, if a parent questions why her daughter or son needs the vaccine, a directed but not overly lengthy response of the importance of this vaccine in preventing cervical and other cancers will inform the parent and teen, and is generally well received. Often, asking if an older family member or friend has suffered with cervical cancer will allow the parent to acknowledge that this is a reality that they do not want their teen to experience. When we fail to recommend the HPV series or say its fine "to wait" because of an inability and unwillingness to raise the link of acquisition of HPV and sexual activity, an opportunity is missed.
The media has done much to promote positive health behaviors, but sadly, parents often recall only the negatives associated with the HPV vaccine. Both clinicians and parents must repurpose themselves to protecting children and adolescents. Parents and youth rely on their pediatrician's recommendations and knowledge. In brief, pediatricians must pin a Post It note to their EHR to cue them to educate, but then vaccinate! In short, "Do Ask, Do Tell." No opportunity to protect our youth should be lost!
Dr. Susan Jay is program director of adolescent health and medicine at Children's Hospital of Wisconsin in Wauwatosa, and a professor of pediatrics at the Medical College of Wisconsin. Dr. Jay has no relevant financial disclosures.
*Correction 8/5/2013: The vaccine was misstated as DTap; teens receive Tdap.
This story was updated. 8/5/2013
Clinicians can do more to improve human papillomavirus vaccination coverage rates among adolescent females, including emphasizing to parents that the vaccine prevents cancer, according to Dr. Thomas Frieden, the director of the Centers for Disease Control and Prevention.
In 2011, only 35% of girls aged 13-17 years had received the full three doses of the HPV vaccine, which dropped slightly in 2012, which is "a huge disappointment," Dr. Frieden said during a July 25 telebriefing. The proportion of adolescent girls who received at least one, two, and the full three-dose HPV vaccine series increased every year from 2007 to 2011, but stalled between 2011 and 2012 and remains at unacceptably low levels – while missed opportunities for vaccination in this population have increased markedly, according to the data (MMWR 2013;62:591-5).
But rates can be increased if clinicians take every possible opportunity to vaccinate adolescents with the HPV vaccine, including when they receive other vaccines, he said during the briefing. While administering a three-dose series was thought to be difficult and was behind the low rates, the data indicated that if the HPV vaccine was given every time the adolescents received another vaccine, the completion of the three-dose series could increase to 93%, he said. According to the report, "Every health care visit, whether for back-to-school evaluations or acute problems, should be used to assess teenagers’ immunization status and provide recommended vaccines if indicated."
"If we get the three-dose series to 80%, an estimated 53,000 cases of cervical cancer could be prevented over the lifetimes of girls aged 12 [years] and younger," Dr. Frieden said.
A three-dose series of Gardasil – the quadrivalent HPV vaccine directed against HPV-16 and -18 (which cause most cervical cancers) and HPV-6 and -11 (which cause most genital warts) – was licensed and recommended by the CDC as a routine vaccine in 2006 for females aged 11-12 years and for females aged 13-26 years who had not been vaccinated. In 2009, the bivalent vaccine Cervarix, directed against HPV-16 and -18, was licensed for use in females aged 10-25 years and added to the recommendation in 2009.
The American Academy of Pediatrics "shares CDC’s disappointment and concern that we are not providing this lifesaving vaccine at the same rates as we do with other immunizations," Dr. Thomas K. McInerny, president of the AAP, said during the briefing.
"Use every opportunity to vaccinate your adolescent patients at both illness and well-child visits," as well as visits for sports physicals, he advised. He also recommended using alerts in electronic medical record systems so vaccination status is reviewed at every patient visit, as well as reminding parents with automated postcards or phone calls, having nurses check vaccine status when they bring patients into the exam room, and implementing standing order policies so that patients can receive vaccines without a physician’s exam or a physician’s order.
The vaccination coverage data were from the National Immunization Survey–Teen (NIS-Teen), which collects data on vaccination among adolescents aged 13-17 years in the 50 states, the District of Columbia, and certain areas of the country, including New York City, and Chicago. Data on HPV vaccination rates among adolescent males will be reported in the future.
In 2007, 25.1% of adolescent girls aged 13-17 years had received at least one dose of the HPV vaccine, which increased to 53% in 2011 and to only 53.8% in 2012. The proportion of adolescent girls who had received the full three-dose series was even lower, increasing from 5.9% in 2007 to 34.8% in 2011 and dropping slightly in 2012 to 33.4%.
The report also provides estimates of missed opportunities for vaccinating adolescent girls, defined as "a health-care encounter occurring on or after a girl’s 11th birthday," and on or after March 23, 2007. Missed opportunities increased from 20.8% in 2007 to 84% in 2012. (March 23, 2007, was when the CDC’s Advisory Committee on Immunization Practices (ACIP) recommendation on the quadrivalent vaccine was published).
The survey found that 23% of parents said they did not plan to get their daughters vaccinated in the next year. The most common reason cited by these parents is that they did not believe the vaccine was necessary (19.1%), followed by concerns about safety (13.1%), vaccine not recommended (14.2%), lack of knowledge of the vaccine or the disease (12.6%), and their daughter was not sexually active (10.1%). Cost did not appear to be an issue.
The report also included safety data, based on the estimated 56 million doses of the quadrivalent vaccine distributed in the United States from June 2006 through March 2013, which did not identify any new safety issues associated with the HPV vaccine, Dr. Frieden said. (Safety focused on the quadrivalent vaccine because most of the vaccine used has been this vaccine).
Most – 92.1% – of the 21,194 adverse event reports received by the Vaccine Adverse Event Reporting System (VAERS) were classified as "nonserious," and included syncope, dizziness, nausea, headache, fever, and urticaria. Local effects included injection-site pain, redness and swelling. Only 8% of the reports were classified as serious and included headache, vomiting, fatigue, dizziness, syncope, and generalized weakness.
The report also points out that no serious safety concerns have been identified in three population-based U.S. studies of the quadrivalent vaccine safety, although one observational study since the vaccine was licensed found an increased syncope risk.
Dr. Frieden noted that an increased risk of syncope may not be specific to the HPV vaccine because syncope is associated with other vaccines among adolescents, and that a 15-minute observation period after the vaccine is administered is recommended.
He referred to a report released in June in the Journal of Infectious Diseases, which found that the infections with the HPV types in the quadrivalent HPV vaccine dropped by almost 60% among females aged 14-19 years during the 4-year period after the vaccine became available and was recommended as a routine vaccine. (J. Inf. Dis. 2013; 208:385-93).
In the United States every year, an estimated 14 million people will become infected with HPV and about 26,200 will develop new cancers from HPV: 17,400 of those cancers are among females, 10,300 of which are cervical cancers, and 8,800 of the cancers are among males, of which 6,700 are oropharyngeal cancers.
In 2011, the recommendation for the quadrivalent vaccine was expanded to include boys aged 11-12 years, and for unvaccinated males up to age 26 years.
Clinicians can do more to improve human papillomavirus vaccination coverage rates among adolescent females, including emphasizing to parents that the vaccine prevents cancer, according to Dr. Thomas Frieden, the director of the Centers for Disease Control and Prevention.
In 2011, only 35% of girls aged 13-17 years had received the full three doses of the HPV vaccine, which dropped slightly in 2012, which is "a huge disappointment," Dr. Frieden said during a July 25 telebriefing. The proportion of adolescent girls who received at least one, two, and the full three-dose HPV vaccine series increased every year from 2007 to 2011, but stalled between 2011 and 2012 and remains at unacceptably low levels – while missed opportunities for vaccination in this population have increased markedly, according to the data (MMWR 2013;62:591-5).
But rates can be increased if clinicians take every possible opportunity to vaccinate adolescents with the HPV vaccine, including when they receive other vaccines, he said during the briefing. While administering a three-dose series was thought to be difficult and was behind the low rates, the data indicated that if the HPV vaccine was given every time the adolescents received another vaccine, the completion of the three-dose series could increase to 93%, he said. According to the report, "Every health care visit, whether for back-to-school evaluations or acute problems, should be used to assess teenagers’ immunization status and provide recommended vaccines if indicated."
"If we get the three-dose series to 80%, an estimated 53,000 cases of cervical cancer could be prevented over the lifetimes of girls aged 12 [years] and younger," Dr. Frieden said.
A three-dose series of Gardasil – the quadrivalent HPV vaccine directed against HPV-16 and -18 (which cause most cervical cancers) and HPV-6 and -11 (which cause most genital warts) – was licensed and recommended by the CDC as a routine vaccine in 2006 for females aged 11-12 years and for females aged 13-26 years who had not been vaccinated. In 2009, the bivalent vaccine Cervarix, directed against HPV-16 and -18, was licensed for use in females aged 10-25 years and added to the recommendation in 2009.
The American Academy of Pediatrics "shares CDC’s disappointment and concern that we are not providing this lifesaving vaccine at the same rates as we do with other immunizations," Dr. Thomas K. McInerny, president of the AAP, said during the briefing.
"Use every opportunity to vaccinate your adolescent patients at both illness and well-child visits," as well as visits for sports physicals, he advised. He also recommended using alerts in electronic medical record systems so vaccination status is reviewed at every patient visit, as well as reminding parents with automated postcards or phone calls, having nurses check vaccine status when they bring patients into the exam room, and implementing standing order policies so that patients can receive vaccines without a physician’s exam or a physician’s order.
The vaccination coverage data were from the National Immunization Survey–Teen (NIS-Teen), which collects data on vaccination among adolescents aged 13-17 years in the 50 states, the District of Columbia, and certain areas of the country, including New York City, and Chicago. Data on HPV vaccination rates among adolescent males will be reported in the future.
In 2007, 25.1% of adolescent girls aged 13-17 years had received at least one dose of the HPV vaccine, which increased to 53% in 2011 and to only 53.8% in 2012. The proportion of adolescent girls who had received the full three-dose series was even lower, increasing from 5.9% in 2007 to 34.8% in 2011 and dropping slightly in 2012 to 33.4%.
The report also provides estimates of missed opportunities for vaccinating adolescent girls, defined as "a health-care encounter occurring on or after a girl’s 11th birthday," and on or after March 23, 2007. Missed opportunities increased from 20.8% in 2007 to 84% in 2012. (March 23, 2007, was when the CDC’s Advisory Committee on Immunization Practices (ACIP) recommendation on the quadrivalent vaccine was published).
The survey found that 23% of parents said they did not plan to get their daughters vaccinated in the next year. The most common reason cited by these parents is that they did not believe the vaccine was necessary (19.1%), followed by concerns about safety (13.1%), vaccine not recommended (14.2%), lack of knowledge of the vaccine or the disease (12.6%), and their daughter was not sexually active (10.1%). Cost did not appear to be an issue.
The report also included safety data, based on the estimated 56 million doses of the quadrivalent vaccine distributed in the United States from June 2006 through March 2013, which did not identify any new safety issues associated with the HPV vaccine, Dr. Frieden said. (Safety focused on the quadrivalent vaccine because most of the vaccine used has been this vaccine).
Most – 92.1% – of the 21,194 adverse event reports received by the Vaccine Adverse Event Reporting System (VAERS) were classified as "nonserious," and included syncope, dizziness, nausea, headache, fever, and urticaria. Local effects included injection-site pain, redness and swelling. Only 8% of the reports were classified as serious and included headache, vomiting, fatigue, dizziness, syncope, and generalized weakness.
The report also points out that no serious safety concerns have been identified in three population-based U.S. studies of the quadrivalent vaccine safety, although one observational study since the vaccine was licensed found an increased syncope risk.
Dr. Frieden noted that an increased risk of syncope may not be specific to the HPV vaccine because syncope is associated with other vaccines among adolescents, and that a 15-minute observation period after the vaccine is administered is recommended.
He referred to a report released in June in the Journal of Infectious Diseases, which found that the infections with the HPV types in the quadrivalent HPV vaccine dropped by almost 60% among females aged 14-19 years during the 4-year period after the vaccine became available and was recommended as a routine vaccine. (J. Inf. Dis. 2013; 208:385-93).
In the United States every year, an estimated 14 million people will become infected with HPV and about 26,200 will develop new cancers from HPV: 17,400 of those cancers are among females, 10,300 of which are cervical cancers, and 8,800 of the cancers are among males, of which 6,700 are oropharyngeal cancers.
In 2011, the recommendation for the quadrivalent vaccine was expanded to include boys aged 11-12 years, and for unvaccinated males up to age 26 years.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major finding: Missed opportunities increased from 20.8% in 2007 to 84% in 2012.
Data source: National Immunization Survey–Teen (NIS-Teen) survey results from 2007 to 2012
Disclosures: CDC did not provide any disclosures.