User login
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10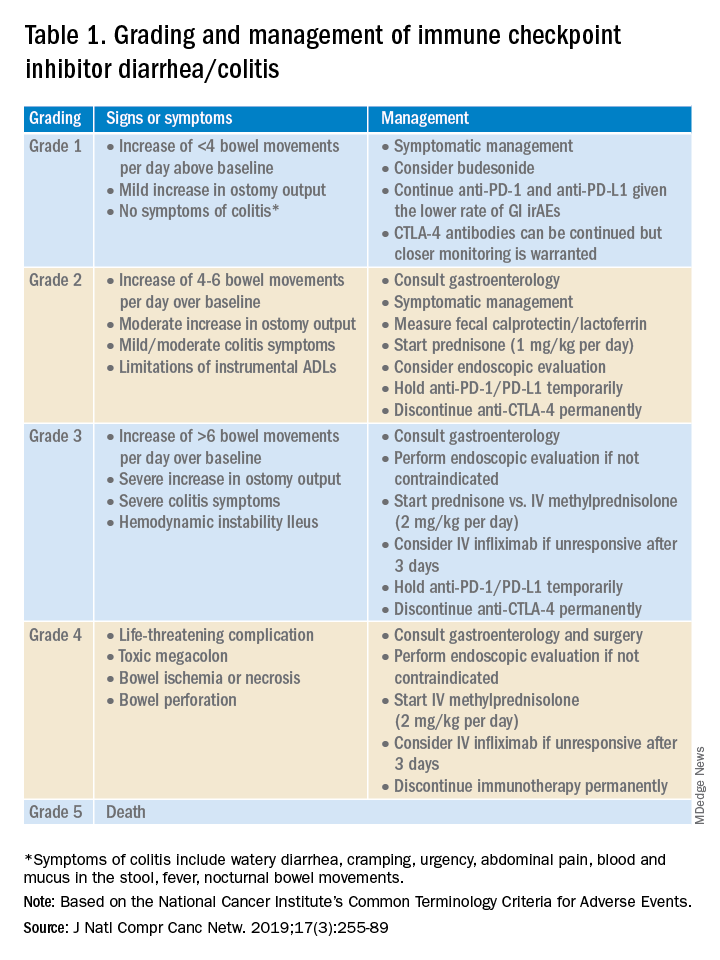
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.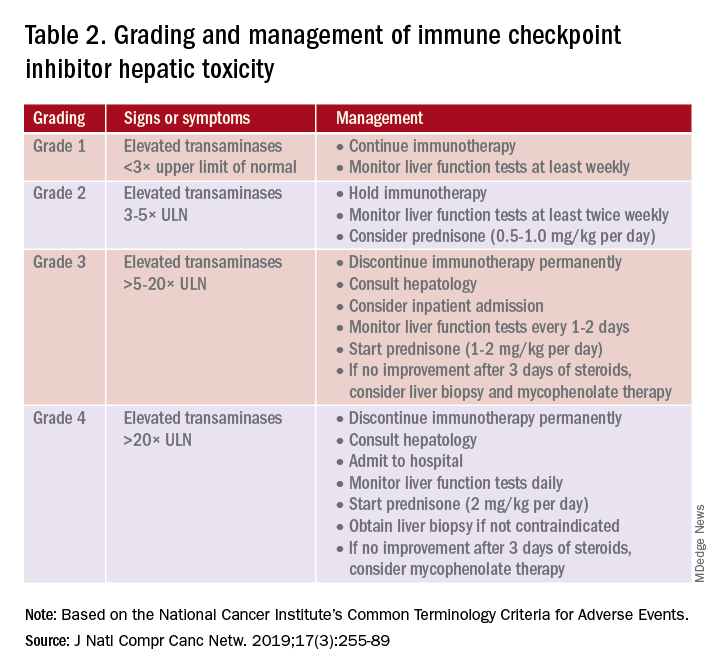
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14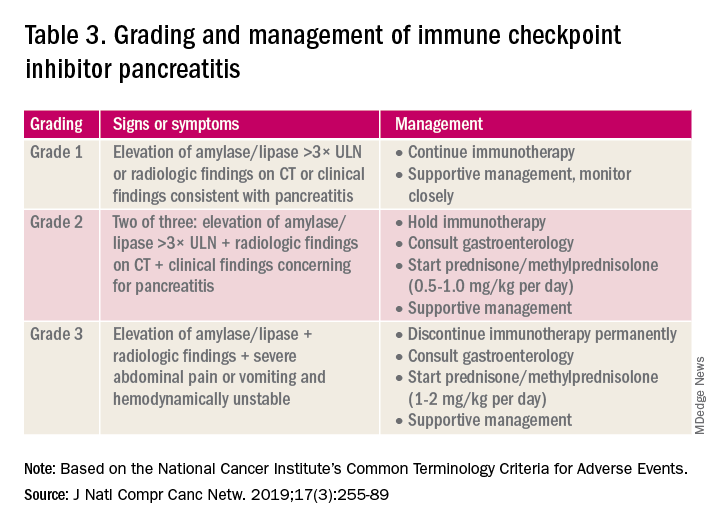
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.


