User login
For MD-IQ use only
Latest COVID-19 Shot May Cut Severe Outcomes in Veterans
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among US veterans, same-day receipt of both the 2024-2025 COVID19 vaccine and the influenza vaccine was associated with lower risks for emergency department visits, hospitalizations, and deaths compared with receipt of the influenza vaccine alone.
METHODOLOGY:
- Researchers conducted an observational study to assess the effectiveness of the 2024-2025 COVID-19 vaccine by comparing veterans who received both the COVID-19 and influenza vaccines on the same day with those who received only the influenza vaccine between September 3 and December 31, 2024.
- Data on participants (mean age, approximately 71.5 years; approximately 92% men) were sourced from electronic health records of the Department of Veterans Affairs and included 164,132 veterans who received both vaccines vs 131,839 who received only the seasonal influenza vaccine, with a follow-up duration of 180 days.
- The vaccines used were mainly the 2024-2025 mRNA COVID19 vaccines: Moderna mRNA1273, Pfizer BNT162b2, and the highdose trivalent 2024-2025 seasonal influenza vaccine.
- Primary outcomes were COVID-19-associated emergency department visits, hospitalizations, and deaths.
TAKEAWAY:
- Receipt of both the COVID-19 and influenza vaccines was associated with a lower risk for COVID-19-associated emergency department visits compared with receipt of the influenza vaccine alone, resulting in a vaccine effectiveness of 29.3% and a risk difference of 18.3 per 10,000 persons (95% CI, 10.8-27.6).
- Similarly, COVID-19 vaccine effectiveness was 39.2% (95% CI, 21.6-54.5) against COVID-19-associated hospitalizations, with a risk difference of 7.5 per 10,000 persons (95% CI, 3.4-13.0).
- For COVID-19-associated deaths, vaccine effectiveness was 64% (95% CI, 23.0-85.8), with a risk difference of 2.2 per 10,000 persons (95% CI, 0.5-6.9).
- Benefits were consistent across age groups (< 65, 65-75, and > 75 years) and among people with various comorbidities, including cardiovascular disease and immunocompromised status.
IN PRACTICE:
“The evidence may help inform ongoing discussions about the value of COVID-19 vaccines in the current epidemiologic landscape,” the authors wrote.
SOURCE:
The study was led by Miao Cai, PhD , Research and Development Service, Veterans Affairs St. Louis Health Care System, and the Veterans Research and Education Foundation of St. Louis, Missouri. It was published online in The New England Journal of Medicine .
LIMITATIONS:
The demographic composition of the cohort — predominantly older, White, male veterans — may limit the generalizability of the study. Although numerous covariates were adjusted for, residual confounding could not be fully ruled out. Safety and variantspecific effectiveness were not assessed.
DISCLOSURES:
The study was supported by a grant from the Department of Veterans Affairs. Two authors disclosed consulting for Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Targeted Osteoporosis Program May Benefit At-Risk Older Men
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
U.S. Health Chief Kennedy Targets Vaccine Injury Compensation Program
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
Rurality and Age May Shape Phone-Only Mental Health Care Access Among Veterans
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Searching for the Optimal CRC Surveillance Test
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert

Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.
Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.



Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.



Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Practice Points
- It is important to consider photodermatoses in patients presenting with a rash that is restricted to light-exposed areas of the skin, such as the arms, legs, neck, and face.
- The mainstay of treatment consists of topical corticosteroids. Oral antihistamines should not be heavily relied on, but short-term oral steroids may be considered for rapid improvement if symptoms are severe.
- It is important to note that, much like in contact dermatitis, the underlying photoallergy causing photocontact dermatitis will persist for a lifetime.
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1
Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
A 78-year-old woman was referred to dermatology from the cardiology clinic with concerns of a nonhealing, scablike lesion on the left chest over the implantation site of a dual-chamber permanent pacemaker (PPM). Eight months prior, the patient underwent successful PPM implantation for symptomatic bradycardia and second-degree atrioventricular block. Her cardiologists subsequently noticed an oozing crusting scab at the site of implantation and eventually referred her to dermatology with concerns for squamous cell carcinoma. Physical examination at the current presentation revealed an exophytic serous crust overlying the PPM implantation site on the left chest.
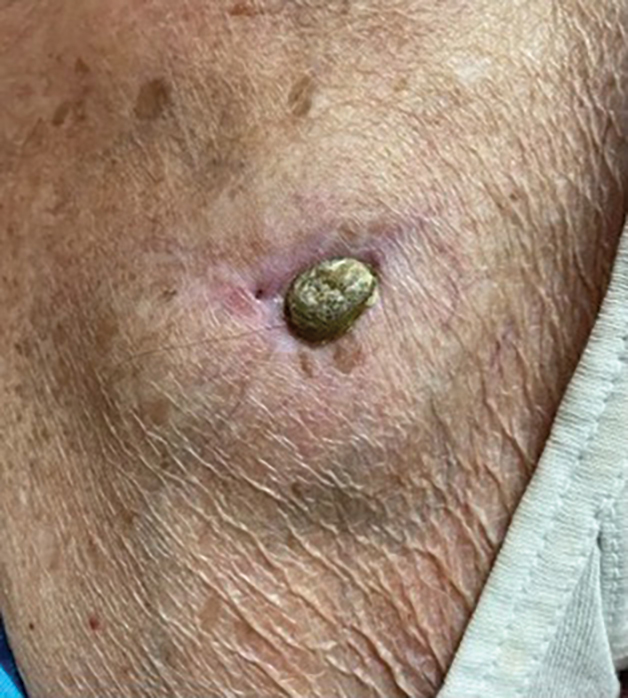
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).


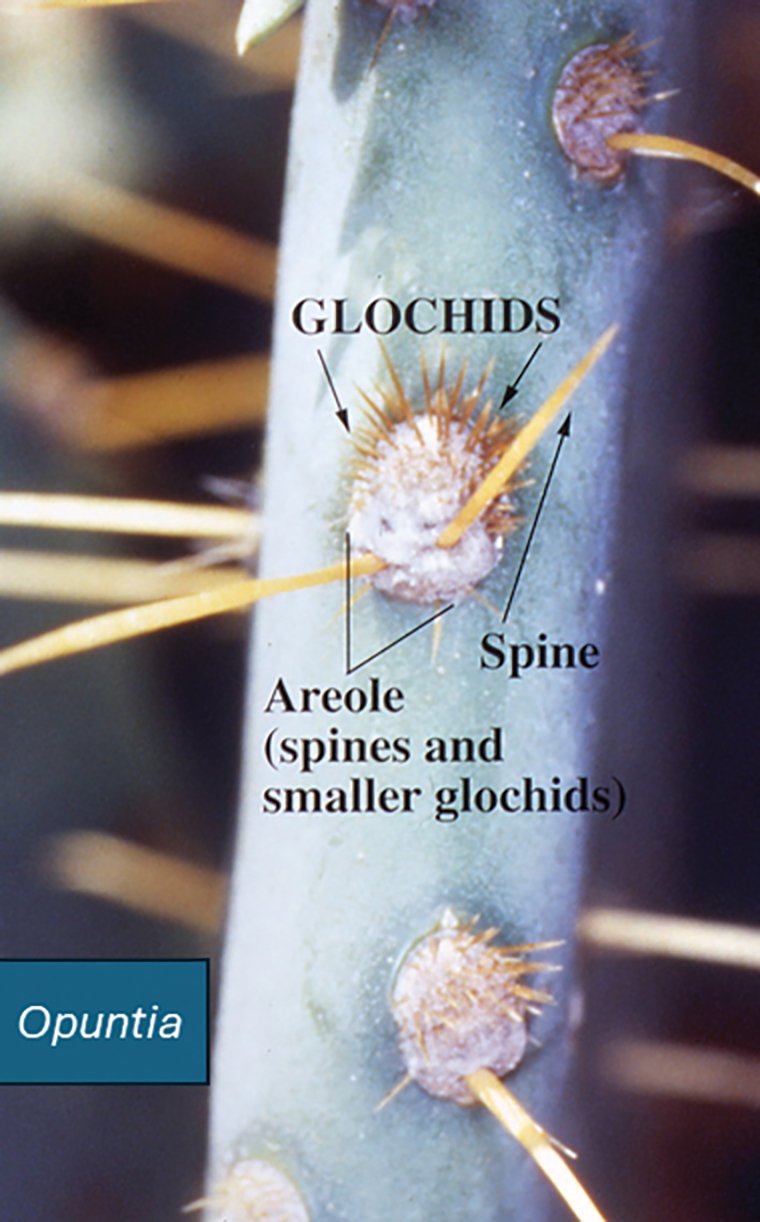
Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Practice Points
- Prickly pear cacti have fine spines that must be removed via scorching or mechanical means before the fruit can be handled safely.
- Prickly pear spines that become embedded in the skin are associated with local and systemic inflammatory conditions as well as allergic contact dermatitis.
- Preclinical studies have suggested that extracts of the prickly pear cactus could be used in medicine for their anti-inflammatory effects.
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.
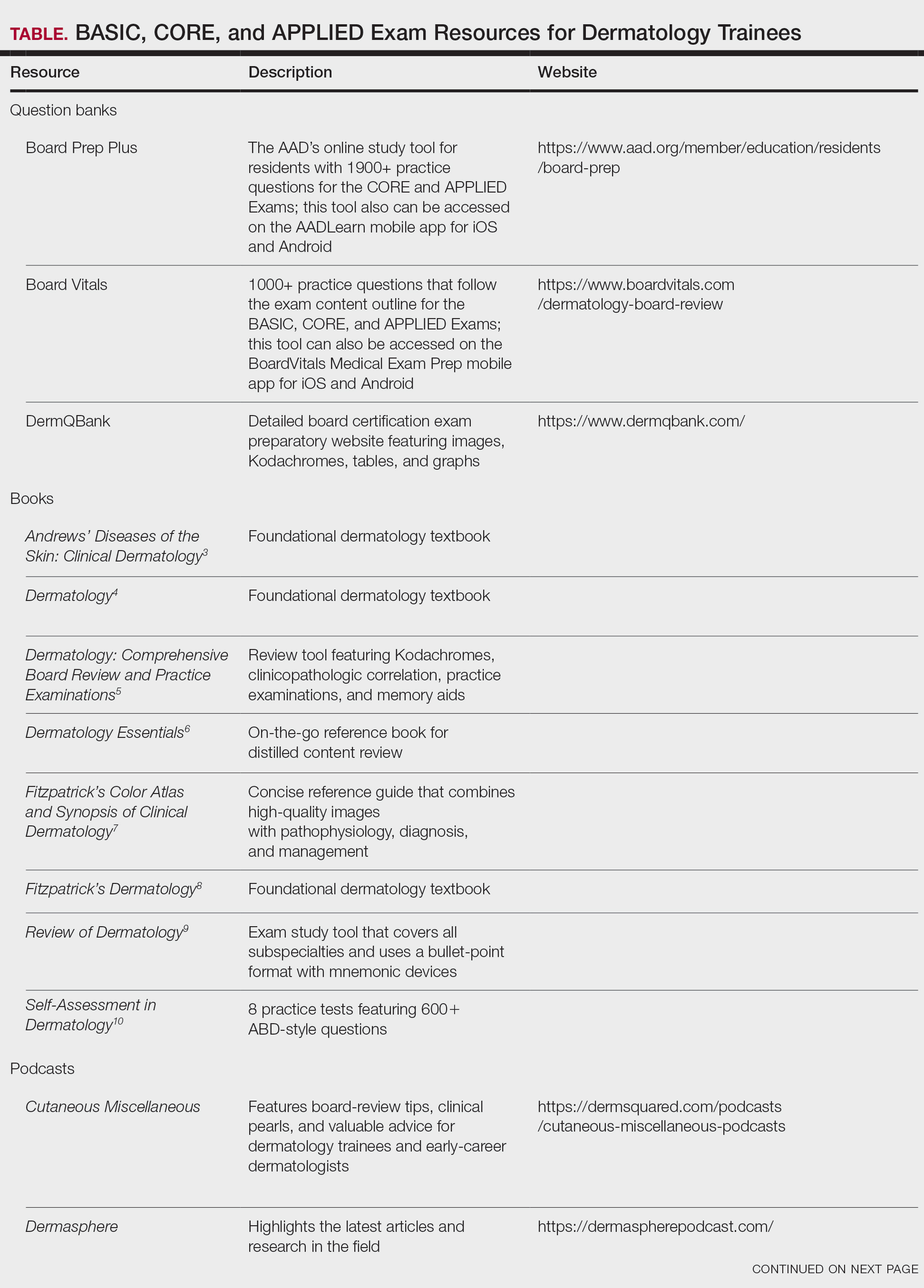
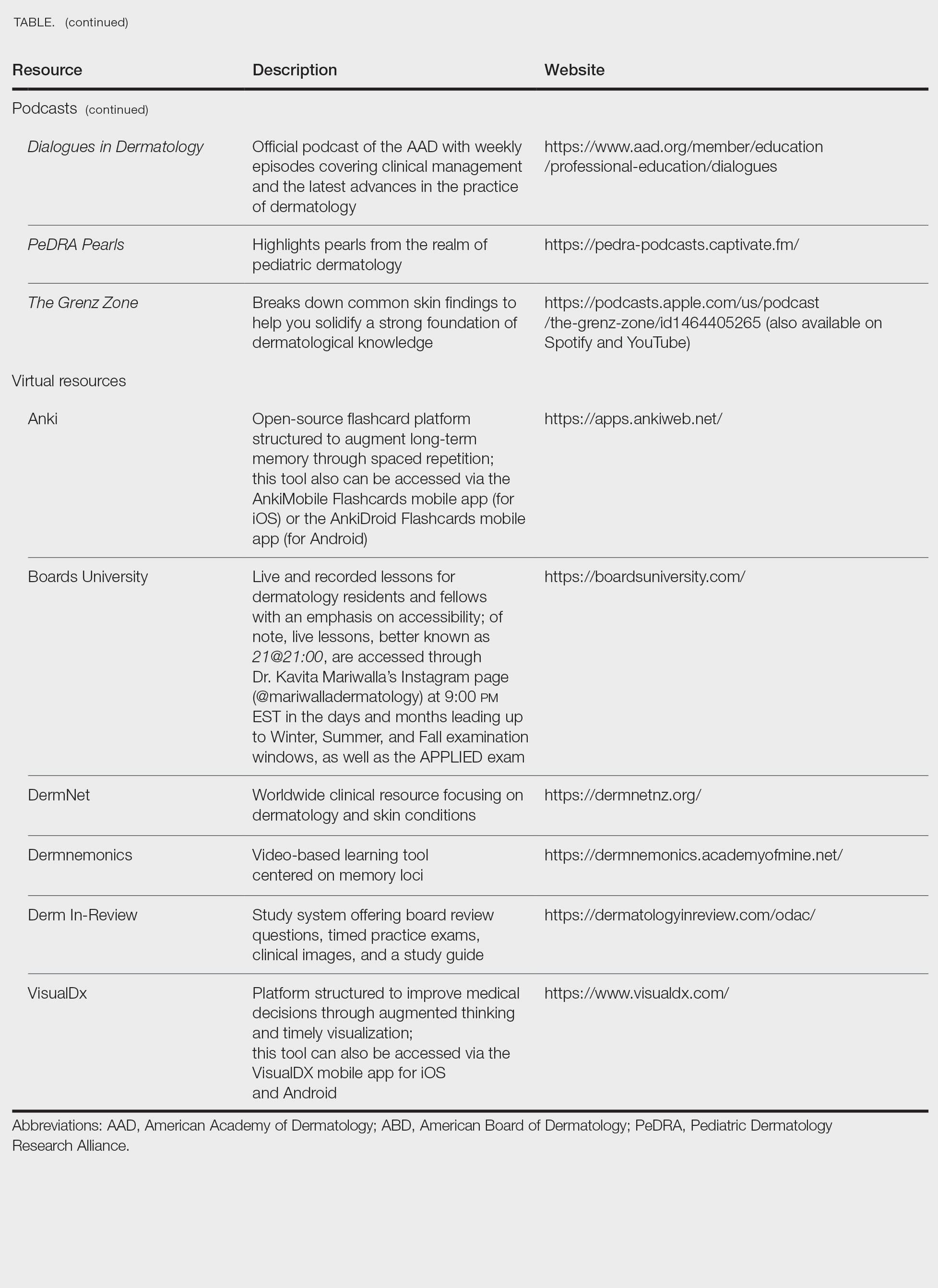
Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.


Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.


Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Practice Points
- To become a board-certified dermatologist, one must complete the American Board of Dermatology Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, followed by 4 CORE Exam modules and a final APPLIED Exam following residency completion.
- When it comes to studying, there are more resources available than you will have time to explore fully. With so many options available, it is crucial to prioritize the ones that best match your learning style.
- A comprehensive study strategy begins during your first year of residency and appropriately intensifies in the months leading up to the exams. Make sure to cultivate test day strategies to help you stay calm, focused, and in the zone.
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2
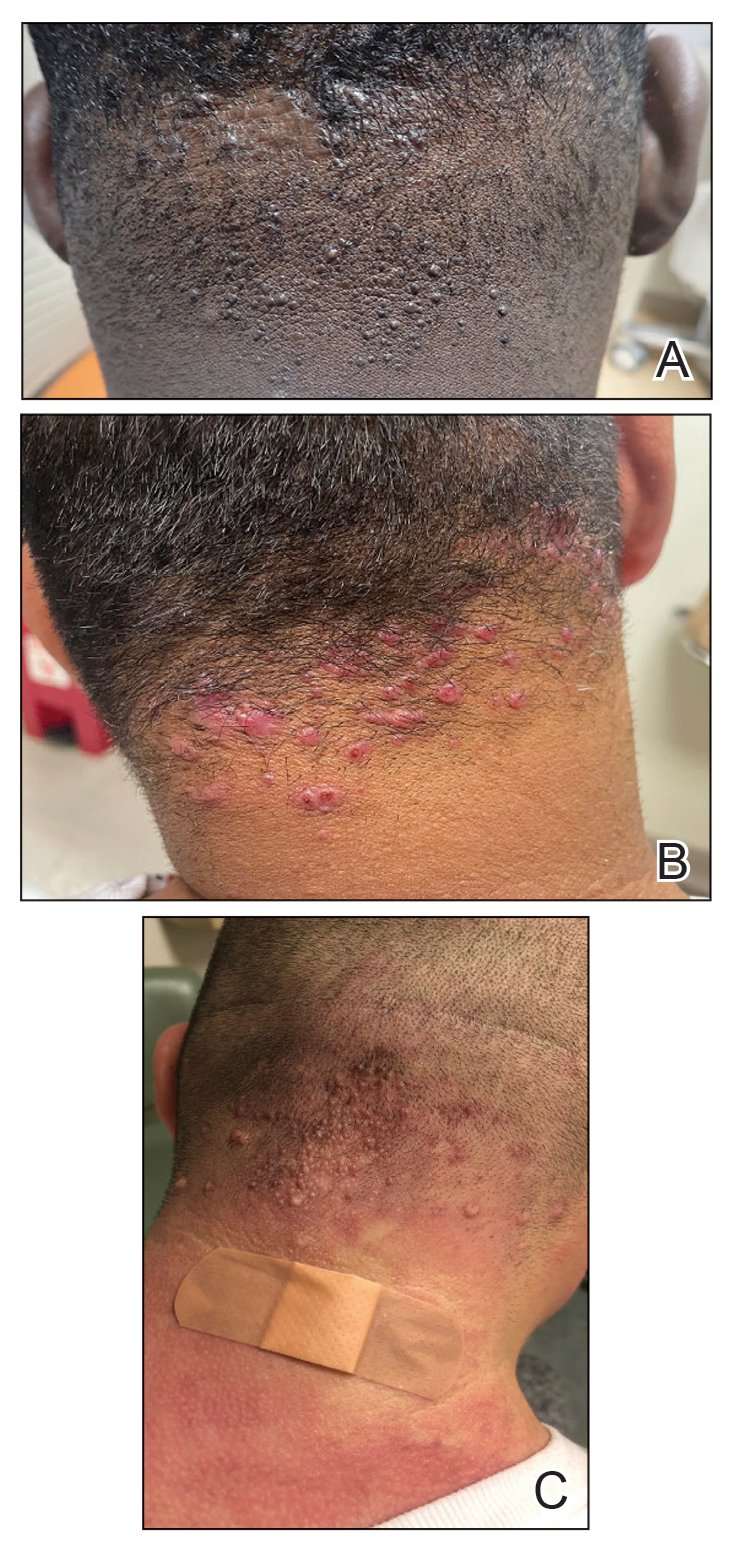
In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.
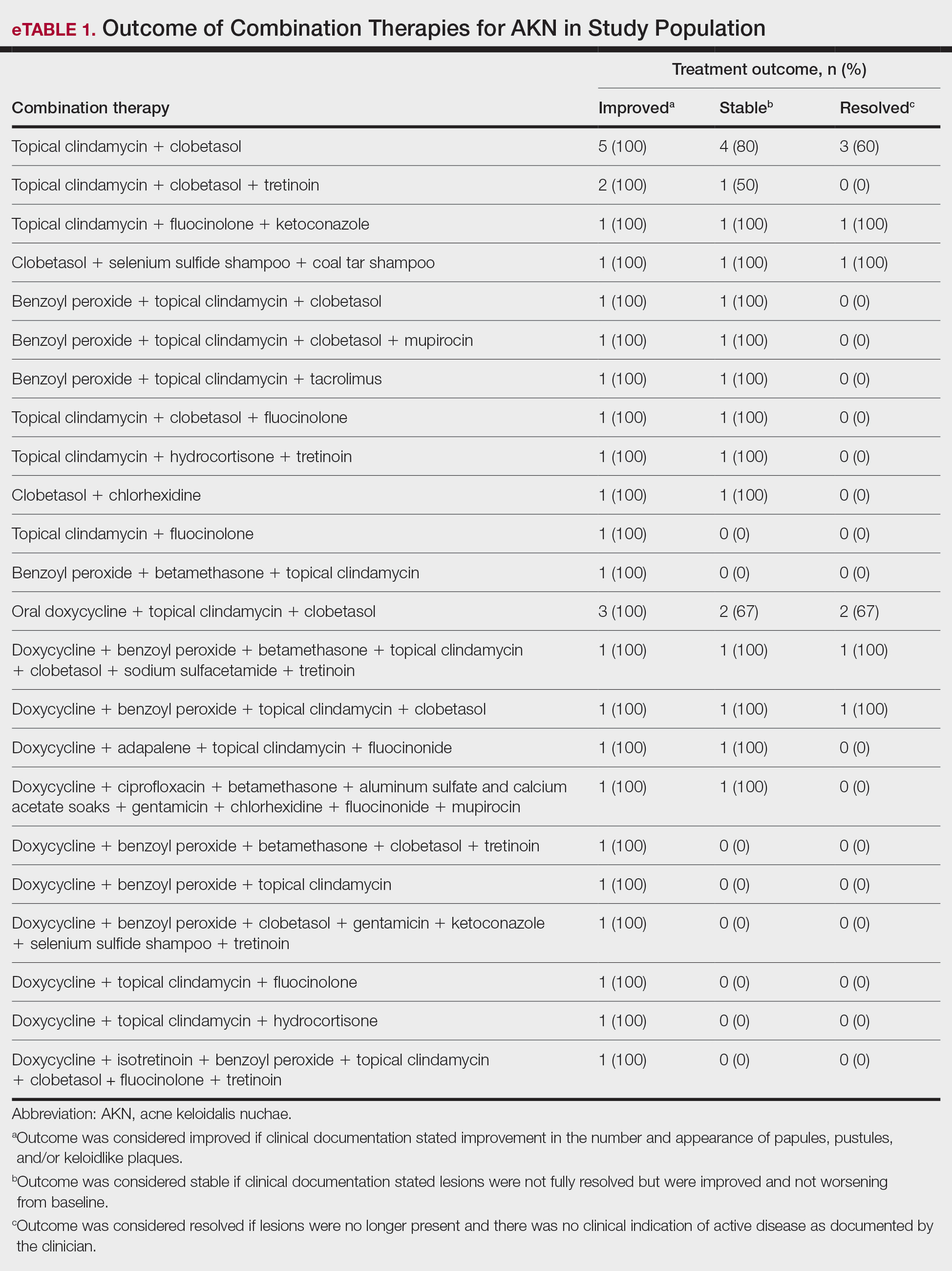
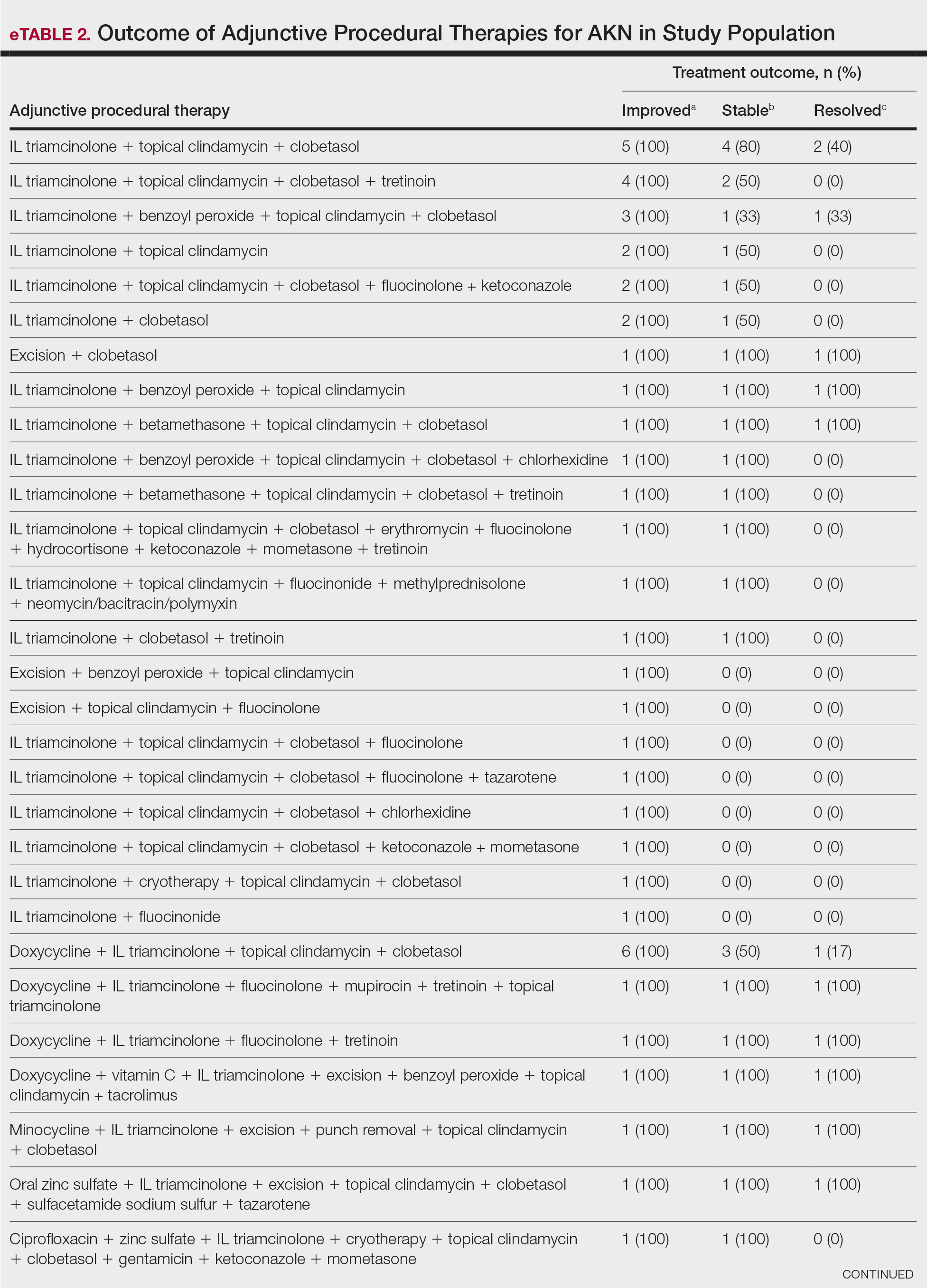
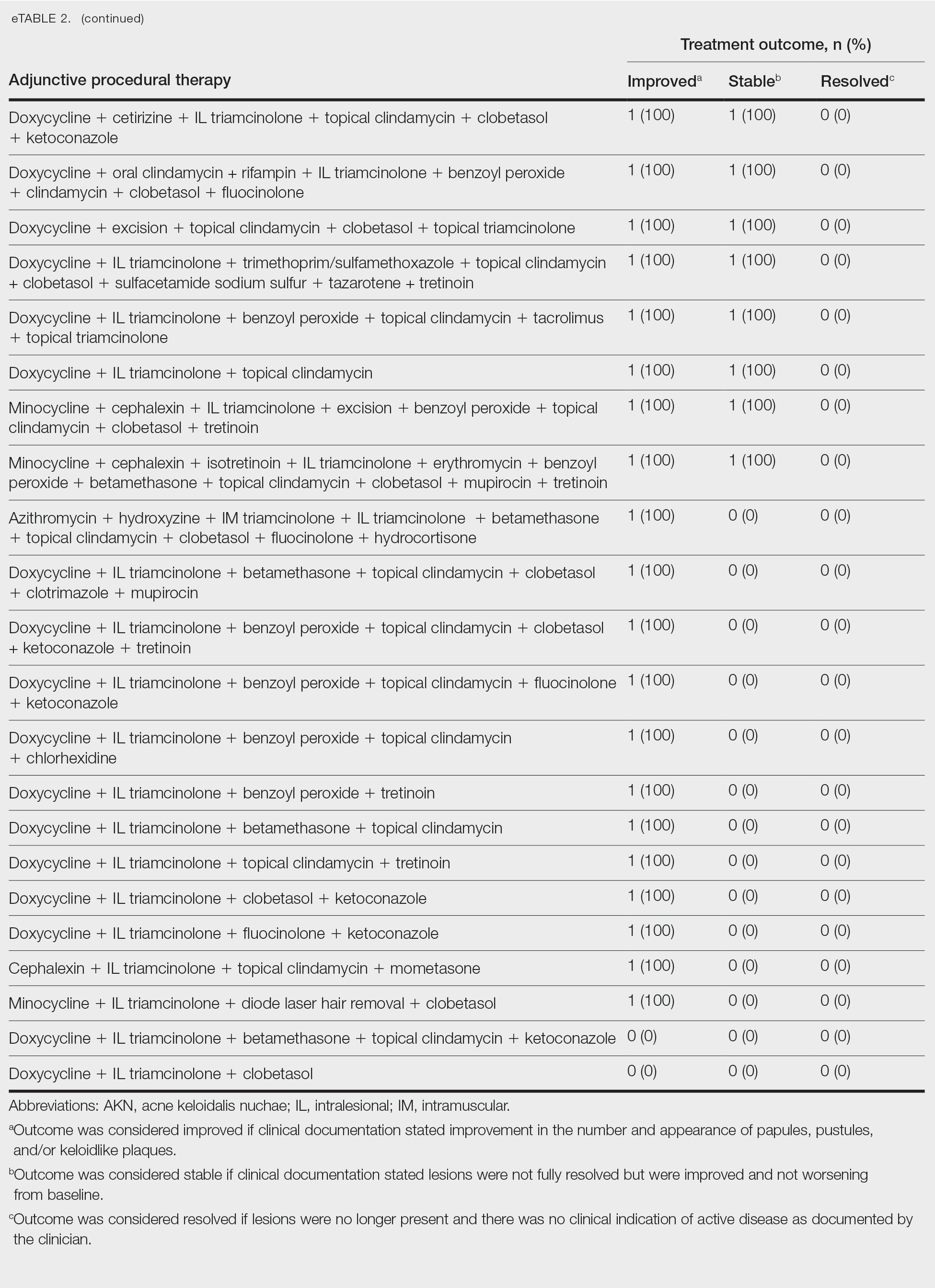
Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
PRACTICE POINTS
- Acne keloidalis nuchae (AKN) is a rare inflammatory skin disease that manifests with papules, pustules, and plaques on the occipital scalp.
- Initial treatment for patients with mild to moderate AKN disease most commonly is topical clindamycin and clobetasol; patients with moderate to severe AKN disease may require adjunctive treatment with oral doxycycline and/or intralesional triamcinolone.
- Combination therapy that targets the multifactorial pathophysiology of AKN (inflammatory, infectious, and traumatic) is most efficacious overall.
- The majority of patients experience improvement of AKN with treatment, but full resolution is less common.