User login
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
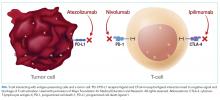
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
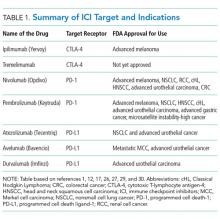
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
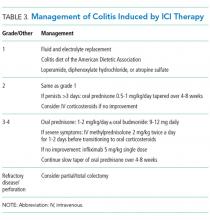
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
© 2018 Society of Hospital Medicine