User login
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
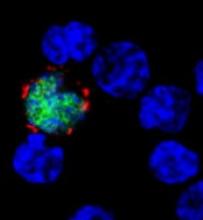
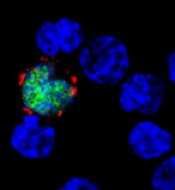
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.
The US Food and Drug Administration (FDA) has granted fast track designation to CMD-003 (baltaleucel-T) for patients with relapsed/refractory lymphoma and post-transplant lymphoproliferative disease associated with Epstein-Barr virus (EBV).
CMD-003 consists of patient-derived T cells that have been activated to kill malignant cells expressing antigens associated with EBV.
The T cells specifically target 4 EBV epitopes—LMP1, LMP2, EBNA, and BARF1.
CMD-003 is being developed by Cell Medica and the Baylor College of Medicine with funding provided, in part, by the Cancer Prevention and Research Institute of Texas.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
CMD-003-related research
CMD-003 is currently under investigation in the phase 2 CITADEL trial for patients with extranodal natural killer T-cell lymphoma and the phase 2 CIVIC trial for patients with EBV-associated diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disease.
Researchers have not published results from any trials of CMD-003, but they have published results with EBV-specific T-cell products related to CMD-003.
In one study, published in the Journal of Clinical Oncology in 2014, researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of the patients died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery.