User login
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
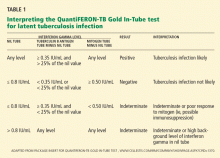
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
KEY POINTS
- Prior vaccination with bacille Calmette-Guérin can cause the results of skin testing to be falsely positive, but it does not affect interferon-gamma-release assays.
- In 2005, the US Centers for Disease Control and Prevention recommended that interferon-gamma-release assays be used in all situations in which skin testing is currently used. Updated guidelines were published on June 25, 2010.
- Successful implementation of interferon-gamma-release assay testing requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians.