User login
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
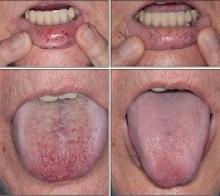
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
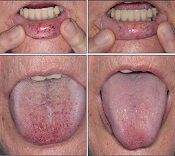
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()