User login
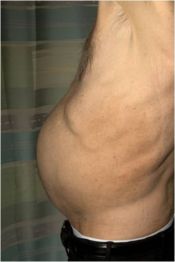
CHICAGO—Two phase 3 studies demonstrate the effectiveness of the investigational Janus kinase (JAK) inhibitor INC424 (ruxolitinib) in treating patients with myelofibrosis (MF), according to presentations at the 2011 ASCO Annual Meeting.
Ruxolitinib has the potential to change the treatment landscape in MF, said Alessandro Vannucchi, MD, of the University of Florence, who reported the results of the COMFORT-II trial.
Srdan Verostovsek, MD, PhD, of the MD Anderson Cancer Center, presented results from COMFORT-I.
COMFORT-II
In the COMFORT-II trial, ruxolitinib produced a volumetric spleen size reduction of 35% or greater in 28.5% of MF patients at 48 weeks. None of the patients receiving best available therapy (BAT) experienced such a reduction, Dr Vannucchi said.
At week 24, 31.9% of ruxolitinib-treated patients had a 35% or greater volumetric spleen size reduction, compared to none of the BAT-treated patients. Ruxolitinib also showed a marked improvement in overall quality of life measures, functioning, and symptoms relative to the BAT arm.
This open-label, phase 3 study included 146 patients randomized to ruxolitinib starting at doses of 15 or 20 mg twice daily and 73 patients randomized to BAT, which was administered at doses and schedules determined by the investigator.
Two-thirds of the BAT patients received at least one medication, and one-third received no medication. The most commonly administered agents were hydroxyurea (47% of patients) and glucocorticoids (16% of patients).
All study participants had intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
The safety profile of ruxolitinib was consistent with previous studies, Dr Vannucchi said. The most common grade 3 or higher adverse events in the ruxolitinib arm were anemia and thrombocytopenia. In the BAT arm, the most common events were anemia, thrombocytopenia, pneumonia, and dyspnea.
COMFORT-I
The COMFORT-I trial enrolled 309 patients with intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. They were randomly assigned to twice-daily oral ruxolitinib (155 patients) or placebo (154 patients).
Ruxolitinib was dosed at 15 mg or 20 mg, depending on the baseline platelet count. Patients in the placebo arm could cross over to the ruxolitinib arm upon disease progression, and nearly a quarter of patients did cross over (11% prior to week 24 and 13% after week 24).
The median follow-up was 32 weeks. A significantly higher proportion of patients on the ruxolitinib arm (41.9%) attained the primary endpoint of at least a 35% reduction in spleen volume after 24 weeks of therapy compared with placebo patients (0.7%), Dr Verstovsek reported.
Ruxolitinib was also more effective than placebo for improving symptom burden, as 45.9% of patients in the ruxolitinib had at least a 50% improvement in symptom burden, compared to 5.3% of placebo-treated patients.
Symptoms that significantly improved with ruxolitinib included abdominal discomfort, pain under the left ribs, early satiety, night sweats, itching, bone or muscle pain, and inactivity.
“Data from the COMFORT studies indicate that ruxolitinib has the potential to significantly improve the current treatment landscape for most patients with MF,” Dr Vannucchi said. ![]()

CHICAGO—Two phase 3 studies demonstrate the effectiveness of the investigational Janus kinase (JAK) inhibitor INC424 (ruxolitinib) in treating patients with myelofibrosis (MF), according to presentations at the 2011 ASCO Annual Meeting.
Ruxolitinib has the potential to change the treatment landscape in MF, said Alessandro Vannucchi, MD, of the University of Florence, who reported the results of the COMFORT-II trial.
Srdan Verostovsek, MD, PhD, of the MD Anderson Cancer Center, presented results from COMFORT-I.
COMFORT-II
In the COMFORT-II trial, ruxolitinib produced a volumetric spleen size reduction of 35% or greater in 28.5% of MF patients at 48 weeks. None of the patients receiving best available therapy (BAT) experienced such a reduction, Dr Vannucchi said.
At week 24, 31.9% of ruxolitinib-treated patients had a 35% or greater volumetric spleen size reduction, compared to none of the BAT-treated patients. Ruxolitinib also showed a marked improvement in overall quality of life measures, functioning, and symptoms relative to the BAT arm.
This open-label, phase 3 study included 146 patients randomized to ruxolitinib starting at doses of 15 or 20 mg twice daily and 73 patients randomized to BAT, which was administered at doses and schedules determined by the investigator.
Two-thirds of the BAT patients received at least one medication, and one-third received no medication. The most commonly administered agents were hydroxyurea (47% of patients) and glucocorticoids (16% of patients).
All study participants had intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
The safety profile of ruxolitinib was consistent with previous studies, Dr Vannucchi said. The most common grade 3 or higher adverse events in the ruxolitinib arm were anemia and thrombocytopenia. In the BAT arm, the most common events were anemia, thrombocytopenia, pneumonia, and dyspnea.
COMFORT-I
The COMFORT-I trial enrolled 309 patients with intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. They were randomly assigned to twice-daily oral ruxolitinib (155 patients) or placebo (154 patients).
Ruxolitinib was dosed at 15 mg or 20 mg, depending on the baseline platelet count. Patients in the placebo arm could cross over to the ruxolitinib arm upon disease progression, and nearly a quarter of patients did cross over (11% prior to week 24 and 13% after week 24).
The median follow-up was 32 weeks. A significantly higher proportion of patients on the ruxolitinib arm (41.9%) attained the primary endpoint of at least a 35% reduction in spleen volume after 24 weeks of therapy compared with placebo patients (0.7%), Dr Verstovsek reported.
Ruxolitinib was also more effective than placebo for improving symptom burden, as 45.9% of patients in the ruxolitinib had at least a 50% improvement in symptom burden, compared to 5.3% of placebo-treated patients.
Symptoms that significantly improved with ruxolitinib included abdominal discomfort, pain under the left ribs, early satiety, night sweats, itching, bone or muscle pain, and inactivity.
“Data from the COMFORT studies indicate that ruxolitinib has the potential to significantly improve the current treatment landscape for most patients with MF,” Dr Vannucchi said. ![]()

CHICAGO—Two phase 3 studies demonstrate the effectiveness of the investigational Janus kinase (JAK) inhibitor INC424 (ruxolitinib) in treating patients with myelofibrosis (MF), according to presentations at the 2011 ASCO Annual Meeting.
Ruxolitinib has the potential to change the treatment landscape in MF, said Alessandro Vannucchi, MD, of the University of Florence, who reported the results of the COMFORT-II trial.
Srdan Verostovsek, MD, PhD, of the MD Anderson Cancer Center, presented results from COMFORT-I.
COMFORT-II
In the COMFORT-II trial, ruxolitinib produced a volumetric spleen size reduction of 35% or greater in 28.5% of MF patients at 48 weeks. None of the patients receiving best available therapy (BAT) experienced such a reduction, Dr Vannucchi said.
At week 24, 31.9% of ruxolitinib-treated patients had a 35% or greater volumetric spleen size reduction, compared to none of the BAT-treated patients. Ruxolitinib also showed a marked improvement in overall quality of life measures, functioning, and symptoms relative to the BAT arm.
This open-label, phase 3 study included 146 patients randomized to ruxolitinib starting at doses of 15 or 20 mg twice daily and 73 patients randomized to BAT, which was administered at doses and schedules determined by the investigator.
Two-thirds of the BAT patients received at least one medication, and one-third received no medication. The most commonly administered agents were hydroxyurea (47% of patients) and glucocorticoids (16% of patients).
All study participants had intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
The safety profile of ruxolitinib was consistent with previous studies, Dr Vannucchi said. The most common grade 3 or higher adverse events in the ruxolitinib arm were anemia and thrombocytopenia. In the BAT arm, the most common events were anemia, thrombocytopenia, pneumonia, and dyspnea.
COMFORT-I
The COMFORT-I trial enrolled 309 patients with intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF. They were randomly assigned to twice-daily oral ruxolitinib (155 patients) or placebo (154 patients).
Ruxolitinib was dosed at 15 mg or 20 mg, depending on the baseline platelet count. Patients in the placebo arm could cross over to the ruxolitinib arm upon disease progression, and nearly a quarter of patients did cross over (11% prior to week 24 and 13% after week 24).
The median follow-up was 32 weeks. A significantly higher proportion of patients on the ruxolitinib arm (41.9%) attained the primary endpoint of at least a 35% reduction in spleen volume after 24 weeks of therapy compared with placebo patients (0.7%), Dr Verstovsek reported.
Ruxolitinib was also more effective than placebo for improving symptom burden, as 45.9% of patients in the ruxolitinib had at least a 50% improvement in symptom burden, compared to 5.3% of placebo-treated patients.
Symptoms that significantly improved with ruxolitinib included abdominal discomfort, pain under the left ribs, early satiety, night sweats, itching, bone or muscle pain, and inactivity.
“Data from the COMFORT studies indicate that ruxolitinib has the potential to significantly improve the current treatment landscape for most patients with MF,” Dr Vannucchi said. ![]()