User login
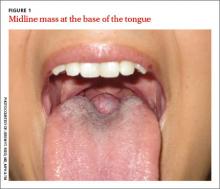
A 26-year-old nonsmoking obese woman presented to our primary care clinic for treatment of a mass at the back of her tongue that was causing intermittent dysphagia and nocturnal choking when she was lying down. She had first noticed the mass 3 years ago; it had been asymptomatic until her recent pregnancy, when its size increased significantly. She denied hemoptysis and dyspnea.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lingual thyroid
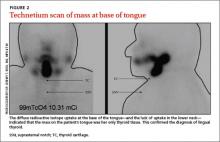
Based on the clinical presentation and location of the mass, and the fact that it had grown larger during pregnancy, we suspected she had lingual thyroid. This is a rare condition that results from the failure of the thyroid to descend from the base of the tongue into the lower neck during early embryogenesis.1 We ordered thyroid function tests and a technetium scan (FIGURE 2), which showed diffuse radioactive isotope uptake at the base of the tongue and no uptake in the lower neck. This indicated that the mass on the tongue was the patient’s only thyroid tissue and thus confirmed the diagnosis.
The incidence of lingual thyroid is 1 in 100,000.2 Women are affected 4 times as often as men, and approximately 75% of patients have no other thyroid tissue.2 Although the condition often is asymptomatic, patients may present with dysphagia, upper airway obstruction, or hemorrhage. Approximately one-third of patients will present with hypothyroidism.3
Differential diagnosis includes squamous cell carcinoma
The differential diagnosis for a posterior, midline tongue mass is broad. Midline masses are typically congenital and lateral ones are often malignant, inflammatory, or infectious. Tongue lesions include oral squamous cell carcinomas (SCCs), mucoceles, and squamous papillomas.
An SCC of the tongue typically presents as a chronic, nonhealing, irregular mass or ulcer that is hard and bleeds easily with manual palpation. The main causes are constant oral mucosal irritants, usually from smoking, chewing tobacco, or alcohol consumption.4 Firm neck lymphadenopathy is common and signifies local metastasis. Men are affected 2 to 4 times more often than women.5,6 Most patients with SCC are >40 years of age.4
Patients with mucoceles often have a history of oral trauma.7 The rapid appearance of a bluish swelling mass is secondary to salivary gland duct disruption as saliva accumulates in surrounding soft tissues. Mucoceles usually occur in patients <20 years of age and are typically <1 cm in diameter.7 They are most commonly found in the lower lip, yet can occur anywhere in the oral cavity. Most will spontaneously rupture and resolve. Definitive treatment is surgical excision.7
Oral squamous papillomas are the most common benign neoplasms of the oral cavity. They typically appear as solitary pink (nonkeratinized) or white (keratinized) lesions on the ventral surface of the tongue, frenulum, or palate.8 They are common in children and adolescents, but can occur at any age. Most arise spontaneously but they also can be caused by direct contact with infected mucosa.
Making the diagnosis
In addition to history and physical exam, diagnosis of lingual thyroid can be confirmed with a radioactive iodine uptake test or a technetium scan. Biopsy is rarely necessary.
Treatment
Lifelong thyroid suppression therapy is warranted for all patients with lingual thyroid9 (strength of recommendation [SOR]: C). In asymptomatic patients this prevents further growth of the lingual thyroid, which often occurs during times of stress such as illness, pregnancy, or puberty.10 In symptomatic patients, thyroid suppression may lead to glandular atrophy and resolution of symptoms.9 Regression in lingual thyroid size is a very slow process. Surgery is reserved for refractory cases and patients with airway obstruction or bleeding.
Our patient’s thyroid-stimulating hormone was significantly elevated at 9.8 mcIU/mL (normal, 0.34-5.60 mcIU/mL) and technetium scanning showed that the lingual thyroid was her only functioning thyroid tissue. It is likely that pregnancy-related stress and increased demand for thyroid hormone caused excessive thyrotropin production. Her lingual thyroid was unable to produce the increased thyroid hormone needed, which resulted in glandular hypertrophy and obstructive symptoms.
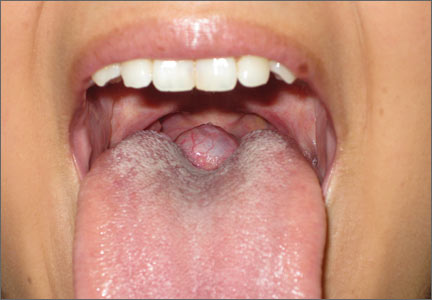
Our patient. After we consulted Endocrinology, we started our patient on levothyroxine 125 mcg/d. Six months later, the mass had shrunk in size and her symptoms had improved. We continue to manage her thyroid suppression and monitor her lingual thyroid size twice a year.
CORRESPONDENCE
Jeremy T. Reed, MD, MPH & TM, Department of Otolaryngology, Carl R. Darnall Army Medical Center, Fort Hood, TX 76544; [email protected]
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality
patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Gupta M, Motwani G. Lingual thyroid. Ear Nose Throat J. 2009;88:E1.
2. Rahbar R, Yoon MJ, Connolly LP, et al. Lingual thyroid in children: a rare clinical entity. Laryngoscope. 2008;118:1174-1179.
3. Neinas FW, Gorman CA, Devine KD, et al. Lingual thyroid: Clinical characteristics of 15 cases. Ann Intern Med. 1973;79:205-210.
4. Fadoo Z, Naz F, Husen Y, et al. Squamous cell carcinoma of tongue in an 11-year-old girl. J Pediatr Hematol Oncol. 2010;32:e199-201.
5. Wildt J, Bundgaard T, Bentzen SM. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1995;20:21-25.
6. Shenoi R, Devrukhkar V, Chaudhuri, et al. Demographic and clinical profile of oral squamous cell carcinoma patients: A retrospective study. Indian J Cancer. 2012;49:21-26.
7. Chi AC, Lambert PR 3rd, Richardson MS, et al. Oral mucoceles: a clinicopathologic review of 1,824 cases, including unusual variants. J Oral Maxillofac Surg. 2011;69:1086-1093.
8. Yeatts D, Bums JC. Common oral mucosal lesions in adults. Am Fam Physician. 1991;44:2043-2050.
9. Kansal P, Sakati N, Rifai A, et al. Lingual thyroid. Diagnosis and treatment. Arch Intern Med. 1987;147:2046-2048.
10. Kalan A, Tariq M. Lingual thyroid gland: clinical evaluation and comprehensive management. Ear Nose Throat J. 1999;78:340-341,345-349.
A 26-year-old nonsmoking obese woman presented to our primary care clinic for treatment of a mass at the back of her tongue that was causing intermittent dysphagia and nocturnal choking when she was lying down. She had first noticed the mass 3 years ago; it had been asymptomatic until her recent pregnancy, when its size increased significantly. She denied hemoptysis and dyspnea.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lingual thyroid
Based on the clinical presentation and location of the mass, and the fact that it had grown larger during pregnancy, we suspected she had lingual thyroid. This is a rare condition that results from the failure of the thyroid to descend from the base of the tongue into the lower neck during early embryogenesis.1 We ordered thyroid function tests and a technetium scan (FIGURE 2), which showed diffuse radioactive isotope uptake at the base of the tongue and no uptake in the lower neck. This indicated that the mass on the tongue was the patient’s only thyroid tissue and thus confirmed the diagnosis.
The incidence of lingual thyroid is 1 in 100,000.2 Women are affected 4 times as often as men, and approximately 75% of patients have no other thyroid tissue.2 Although the condition often is asymptomatic, patients may present with dysphagia, upper airway obstruction, or hemorrhage. Approximately one-third of patients will present with hypothyroidism.3
Differential diagnosis includes squamous cell carcinoma
The differential diagnosis for a posterior, midline tongue mass is broad. Midline masses are typically congenital and lateral ones are often malignant, inflammatory, or infectious. Tongue lesions include oral squamous cell carcinomas (SCCs), mucoceles, and squamous papillomas.
An SCC of the tongue typically presents as a chronic, nonhealing, irregular mass or ulcer that is hard and bleeds easily with manual palpation. The main causes are constant oral mucosal irritants, usually from smoking, chewing tobacco, or alcohol consumption.4 Firm neck lymphadenopathy is common and signifies local metastasis. Men are affected 2 to 4 times more often than women.5,6 Most patients with SCC are >40 years of age.4
Patients with mucoceles often have a history of oral trauma.7 The rapid appearance of a bluish swelling mass is secondary to salivary gland duct disruption as saliva accumulates in surrounding soft tissues. Mucoceles usually occur in patients <20 years of age and are typically <1 cm in diameter.7 They are most commonly found in the lower lip, yet can occur anywhere in the oral cavity. Most will spontaneously rupture and resolve. Definitive treatment is surgical excision.7
Oral squamous papillomas are the most common benign neoplasms of the oral cavity. They typically appear as solitary pink (nonkeratinized) or white (keratinized) lesions on the ventral surface of the tongue, frenulum, or palate.8 They are common in children and adolescents, but can occur at any age. Most arise spontaneously but they also can be caused by direct contact with infected mucosa.
Making the diagnosis
In addition to history and physical exam, diagnosis of lingual thyroid can be confirmed with a radioactive iodine uptake test or a technetium scan. Biopsy is rarely necessary.
Treatment
Lifelong thyroid suppression therapy is warranted for all patients with lingual thyroid9 (strength of recommendation [SOR]: C). In asymptomatic patients this prevents further growth of the lingual thyroid, which often occurs during times of stress such as illness, pregnancy, or puberty.10 In symptomatic patients, thyroid suppression may lead to glandular atrophy and resolution of symptoms.9 Regression in lingual thyroid size is a very slow process. Surgery is reserved for refractory cases and patients with airway obstruction or bleeding.
Our patient’s thyroid-stimulating hormone was significantly elevated at 9.8 mcIU/mL (normal, 0.34-5.60 mcIU/mL) and technetium scanning showed that the lingual thyroid was her only functioning thyroid tissue. It is likely that pregnancy-related stress and increased demand for thyroid hormone caused excessive thyrotropin production. Her lingual thyroid was unable to produce the increased thyroid hormone needed, which resulted in glandular hypertrophy and obstructive symptoms.
Our patient. After we consulted Endocrinology, we started our patient on levothyroxine 125 mcg/d. Six months later, the mass had shrunk in size and her symptoms had improved. We continue to manage her thyroid suppression and monitor her lingual thyroid size twice a year.
CORRESPONDENCE
Jeremy T. Reed, MD, MPH & TM, Department of Otolaryngology, Carl R. Darnall Army Medical Center, Fort Hood, TX 76544; [email protected]
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality
patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 26-year-old nonsmoking obese woman presented to our primary care clinic for treatment of a mass at the back of her tongue that was causing intermittent dysphagia and nocturnal choking when she was lying down. She had first noticed the mass 3 years ago; it had been asymptomatic until her recent pregnancy, when its size increased significantly. She denied hemoptysis and dyspnea.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lingual thyroid
Based on the clinical presentation and location of the mass, and the fact that it had grown larger during pregnancy, we suspected she had lingual thyroid. This is a rare condition that results from the failure of the thyroid to descend from the base of the tongue into the lower neck during early embryogenesis.1 We ordered thyroid function tests and a technetium scan (FIGURE 2), which showed diffuse radioactive isotope uptake at the base of the tongue and no uptake in the lower neck. This indicated that the mass on the tongue was the patient’s only thyroid tissue and thus confirmed the diagnosis.
The incidence of lingual thyroid is 1 in 100,000.2 Women are affected 4 times as often as men, and approximately 75% of patients have no other thyroid tissue.2 Although the condition often is asymptomatic, patients may present with dysphagia, upper airway obstruction, or hemorrhage. Approximately one-third of patients will present with hypothyroidism.3
Differential diagnosis includes squamous cell carcinoma
The differential diagnosis for a posterior, midline tongue mass is broad. Midline masses are typically congenital and lateral ones are often malignant, inflammatory, or infectious. Tongue lesions include oral squamous cell carcinomas (SCCs), mucoceles, and squamous papillomas.
An SCC of the tongue typically presents as a chronic, nonhealing, irregular mass or ulcer that is hard and bleeds easily with manual palpation. The main causes are constant oral mucosal irritants, usually from smoking, chewing tobacco, or alcohol consumption.4 Firm neck lymphadenopathy is common and signifies local metastasis. Men are affected 2 to 4 times more often than women.5,6 Most patients with SCC are >40 years of age.4
Patients with mucoceles often have a history of oral trauma.7 The rapid appearance of a bluish swelling mass is secondary to salivary gland duct disruption as saliva accumulates in surrounding soft tissues. Mucoceles usually occur in patients <20 years of age and are typically <1 cm in diameter.7 They are most commonly found in the lower lip, yet can occur anywhere in the oral cavity. Most will spontaneously rupture and resolve. Definitive treatment is surgical excision.7
Oral squamous papillomas are the most common benign neoplasms of the oral cavity. They typically appear as solitary pink (nonkeratinized) or white (keratinized) lesions on the ventral surface of the tongue, frenulum, or palate.8 They are common in children and adolescents, but can occur at any age. Most arise spontaneously but they also can be caused by direct contact with infected mucosa.
Making the diagnosis
In addition to history and physical exam, diagnosis of lingual thyroid can be confirmed with a radioactive iodine uptake test or a technetium scan. Biopsy is rarely necessary.
Treatment
Lifelong thyroid suppression therapy is warranted for all patients with lingual thyroid9 (strength of recommendation [SOR]: C). In asymptomatic patients this prevents further growth of the lingual thyroid, which often occurs during times of stress such as illness, pregnancy, or puberty.10 In symptomatic patients, thyroid suppression may lead to glandular atrophy and resolution of symptoms.9 Regression in lingual thyroid size is a very slow process. Surgery is reserved for refractory cases and patients with airway obstruction or bleeding.
Our patient’s thyroid-stimulating hormone was significantly elevated at 9.8 mcIU/mL (normal, 0.34-5.60 mcIU/mL) and technetium scanning showed that the lingual thyroid was her only functioning thyroid tissue. It is likely that pregnancy-related stress and increased demand for thyroid hormone caused excessive thyrotropin production. Her lingual thyroid was unable to produce the increased thyroid hormone needed, which resulted in glandular hypertrophy and obstructive symptoms.
Our patient. After we consulted Endocrinology, we started our patient on levothyroxine 125 mcg/d. Six months later, the mass had shrunk in size and her symptoms had improved. We continue to manage her thyroid suppression and monitor her lingual thyroid size twice a year.
CORRESPONDENCE
Jeremy T. Reed, MD, MPH & TM, Department of Otolaryngology, Carl R. Darnall Army Medical Center, Fort Hood, TX 76544; [email protected]
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality
patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Gupta M, Motwani G. Lingual thyroid. Ear Nose Throat J. 2009;88:E1.
2. Rahbar R, Yoon MJ, Connolly LP, et al. Lingual thyroid in children: a rare clinical entity. Laryngoscope. 2008;118:1174-1179.
3. Neinas FW, Gorman CA, Devine KD, et al. Lingual thyroid: Clinical characteristics of 15 cases. Ann Intern Med. 1973;79:205-210.
4. Fadoo Z, Naz F, Husen Y, et al. Squamous cell carcinoma of tongue in an 11-year-old girl. J Pediatr Hematol Oncol. 2010;32:e199-201.
5. Wildt J, Bundgaard T, Bentzen SM. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1995;20:21-25.
6. Shenoi R, Devrukhkar V, Chaudhuri, et al. Demographic and clinical profile of oral squamous cell carcinoma patients: A retrospective study. Indian J Cancer. 2012;49:21-26.
7. Chi AC, Lambert PR 3rd, Richardson MS, et al. Oral mucoceles: a clinicopathologic review of 1,824 cases, including unusual variants. J Oral Maxillofac Surg. 2011;69:1086-1093.
8. Yeatts D, Bums JC. Common oral mucosal lesions in adults. Am Fam Physician. 1991;44:2043-2050.
9. Kansal P, Sakati N, Rifai A, et al. Lingual thyroid. Diagnosis and treatment. Arch Intern Med. 1987;147:2046-2048.
10. Kalan A, Tariq M. Lingual thyroid gland: clinical evaluation and comprehensive management. Ear Nose Throat J. 1999;78:340-341,345-349.
1. Gupta M, Motwani G. Lingual thyroid. Ear Nose Throat J. 2009;88:E1.
2. Rahbar R, Yoon MJ, Connolly LP, et al. Lingual thyroid in children: a rare clinical entity. Laryngoscope. 2008;118:1174-1179.
3. Neinas FW, Gorman CA, Devine KD, et al. Lingual thyroid: Clinical characteristics of 15 cases. Ann Intern Med. 1973;79:205-210.
4. Fadoo Z, Naz F, Husen Y, et al. Squamous cell carcinoma of tongue in an 11-year-old girl. J Pediatr Hematol Oncol. 2010;32:e199-201.
5. Wildt J, Bundgaard T, Bentzen SM. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1995;20:21-25.
6. Shenoi R, Devrukhkar V, Chaudhuri, et al. Demographic and clinical profile of oral squamous cell carcinoma patients: A retrospective study. Indian J Cancer. 2012;49:21-26.
7. Chi AC, Lambert PR 3rd, Richardson MS, et al. Oral mucoceles: a clinicopathologic review of 1,824 cases, including unusual variants. J Oral Maxillofac Surg. 2011;69:1086-1093.
8. Yeatts D, Bums JC. Common oral mucosal lesions in adults. Am Fam Physician. 1991;44:2043-2050.
9. Kansal P, Sakati N, Rifai A, et al. Lingual thyroid. Diagnosis and treatment. Arch Intern Med. 1987;147:2046-2048.
10. Kalan A, Tariq M. Lingual thyroid gland: clinical evaluation and comprehensive management. Ear Nose Throat J. 1999;78:340-341,345-349.