User login
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
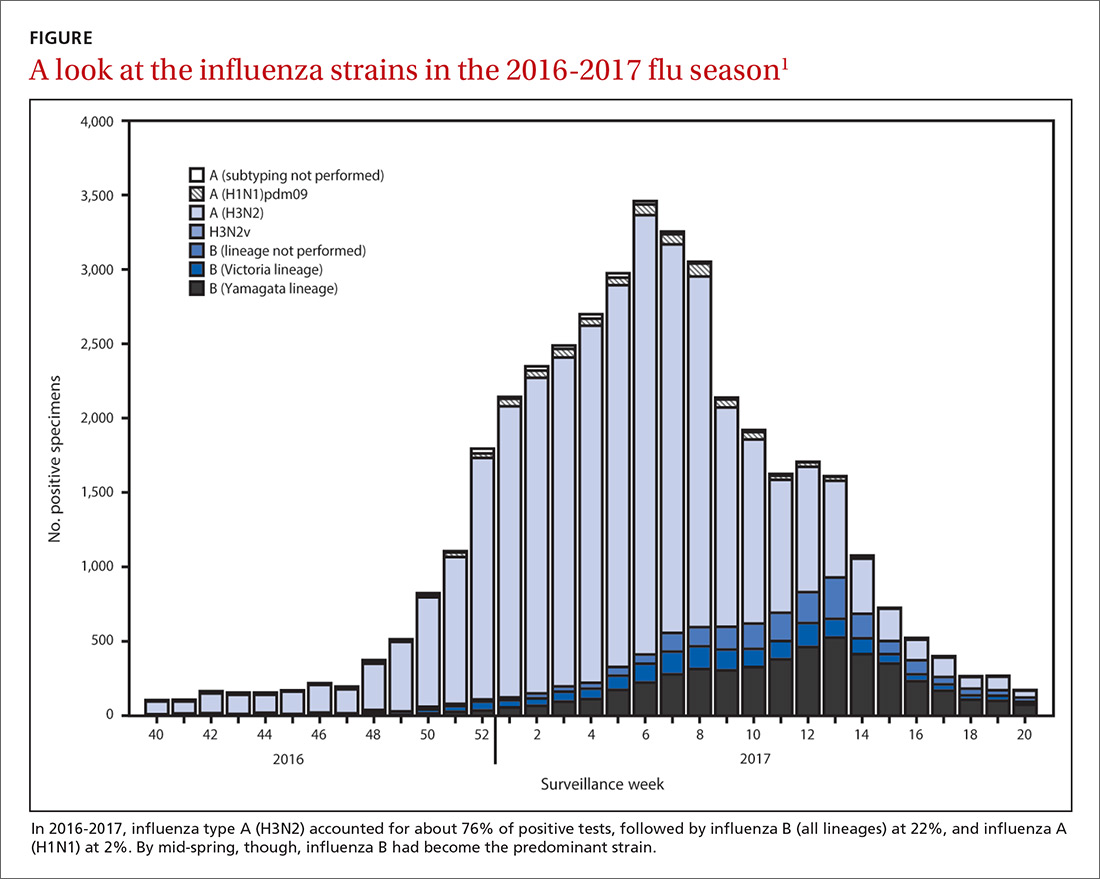
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.
Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
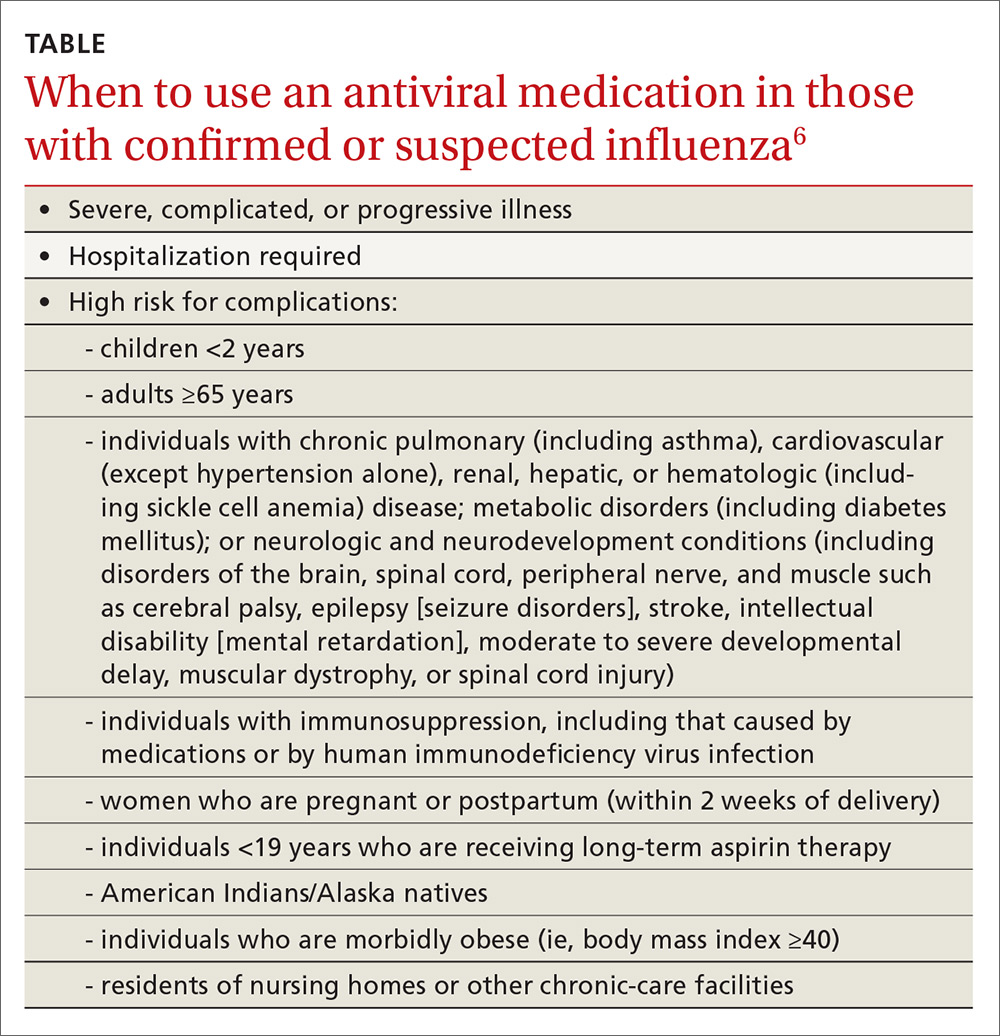
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.
Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.