User login
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

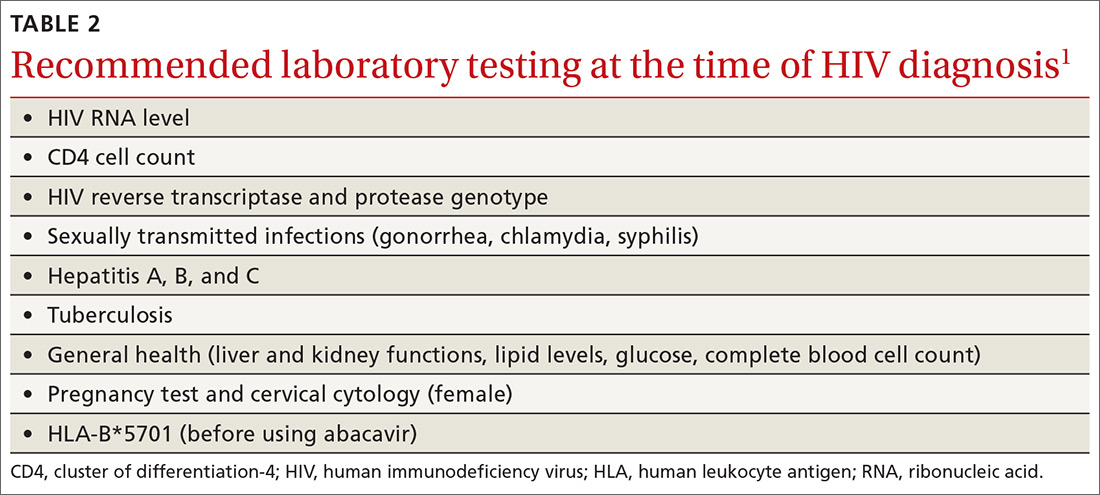
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
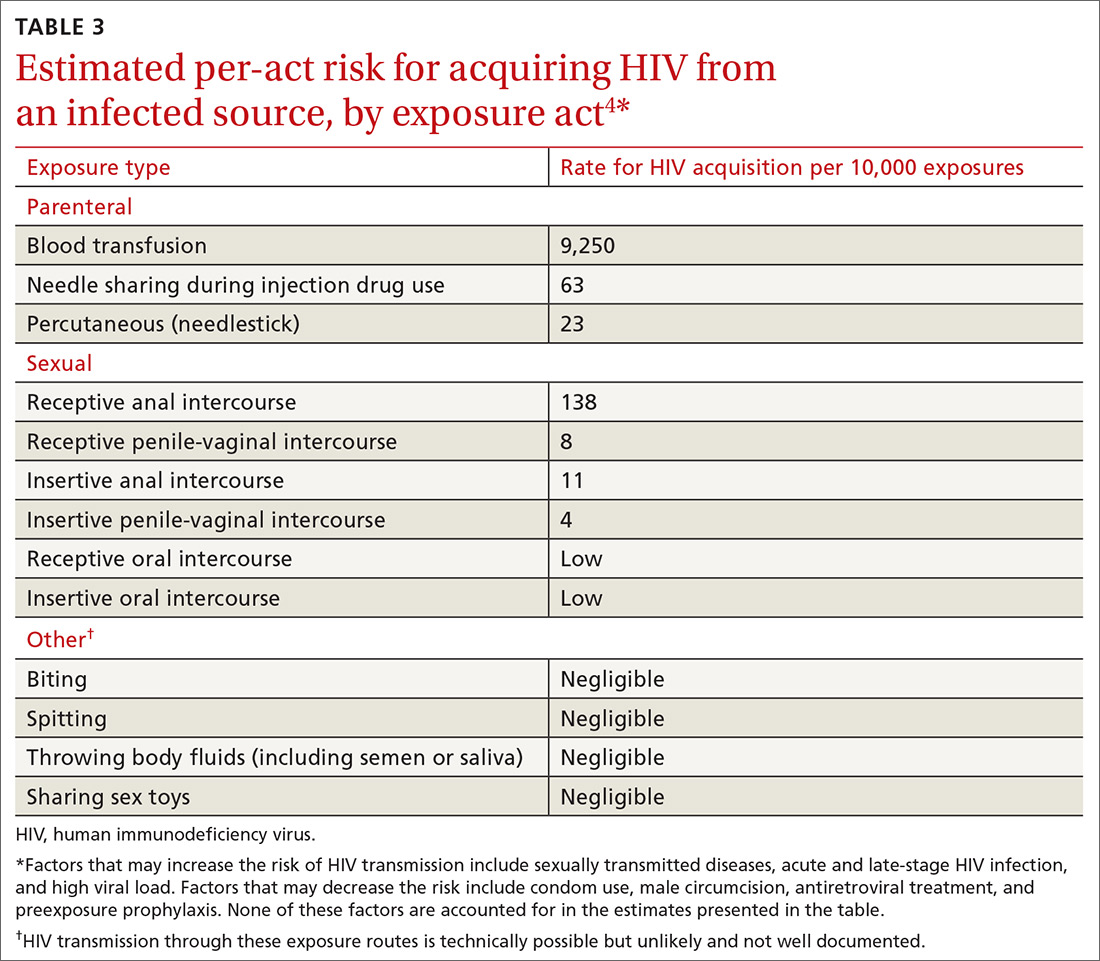
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
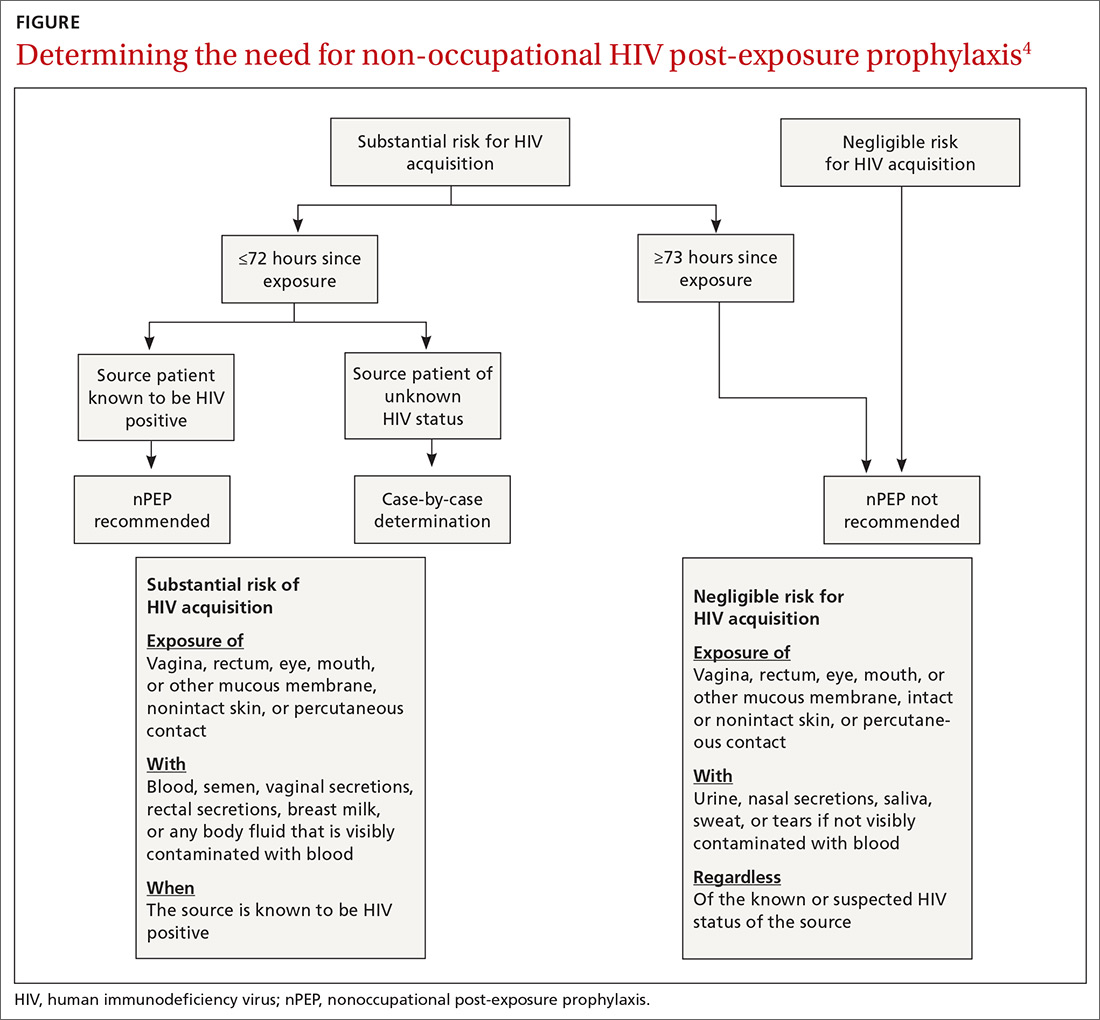
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.