User login
In patients with stable coronary artery disease (CAD), the cornerstone of treatment is medical management to control symptoms such as angina and dyspnea on exertion. But in a select group of patients, percutaneous coronary intervention (PCI) is indicated in addition to medical management. Invasive and noninvasive hemodynamic assessments of coronary artery stenosis in conjunction with anatomic considerations play a role in decision-making and in advising patients on revascularization vs medical management. However, in the case of coronary artery chronic total occlusion (CTO), the decision-making process remains challenging due to limited evidence supporting clinical efficacy of CTO PCI, as well as practical considerations including lower success rates and higher complication rates in comparison with patent-vessel PCI.
CLINICAL VIGNETTE
A 42-year-old man, an avid runner with hyperlipidemia and a strong family history of premature CAD, presents with several months of declining exercise tolerance. His physical examination and electrocardiogram are unremarkable. Myocardial perfusion imaging shows stress-induced ischemia affecting about 20% of the inferolateral myocardium. He is then referred for coronary angiography.
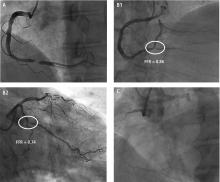
- Panel A: Discrete, high-grade stenosis of the mid-right coronary artery
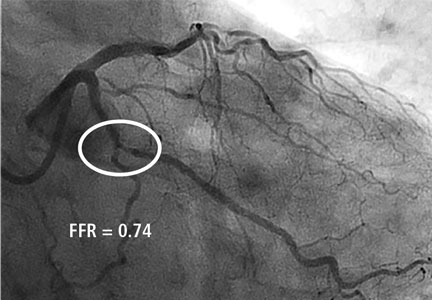
- Panel B: Diffuse, multivessel disease involving the distal right coronary artery (B1) and the proximal left circumflex coronary artery (B2)
- Panel C: Total occlusion of the proximal right coronary artery with extensive left-to-right collaterals.
Treatment based on angiographic findings
In panel A, there is little to debate. The patient is likely to benefit from percutaneous revascularization of the right coronary artery to treat symptoms.
In panels B1 and B2, there is abundant evidence that the hemodynamic assessment of stenosis is superior to a visual estimate in directing PCI.1,2 Hemodynamic assessments including fractional flow reserve (FFR) inform the risk-benefit analysis of percutaneous vs medical treatment of coronary stenosis. In the case of FFR, 0.8 represents an inflection point. The lower FFR values are below 0.8, the greater the benefit of PCI as opposed to medical therapy. Conversely, the greater FFR values are above 0.8, the greater the benefit of medical therapy as opposed to PCI.
However, in panel C, there is significant variability in the data supporting the best treatment strategy for symptomatic patients with CTO.
CORONARY CTO
Coronary CTO is defined as TIMI 0 flow for more than 3 months in an epicardial coronary artery. CTO is not uncommon, seen on 30% of routine coronary angiograms. In the United States, attempt rates of PCI for CTO remain low and have been static at around 12.4%, representing less than 5% of total PCI volume.3 In addition, success rates of CTO PCI are disappointingly low at 59% compared with success rates of patent-vessel PCI at 96%.3 The most frequently cited barriers to CTO PCI are incomplete evidence for efficacy and concerns about safety. Because of the ongoing controversy about the risks and benefits of CTO PCI, it remains a class IIa indication in current American and European practice guidelines.4,5 In addition, these procedures remain technically challenging, and thus variability in local expertise can influence the decision to manage patients medically or refer for CTO PCI.
Patients are often advised that CTO is benign. However, the myocardium affected by a CTO is ischemic. Collateral vessels do not provide adequate flow reserve. FFR data collected from CTOs that were successfully crossed and subsequently interrogated with a pressure wire prior to stenting show that the myocardium supplied by the reconstituted distal bed remains ischemic. This ischemic burden appears to be independent of the size and quality of collaterals.6,7 In addition, a moderate stenosis in a donor coronary artery supplying collateral vessels to a CTO may result in an ischemic FFR as a consequence of coronary “steal” from the donor artery to the collateral vessels. The ischemic FFR in the donor artery can be corrected by treating the recipient CTO vessel.8
Similar to FFR, noninvasive assessment using myocardial perfusion imaging can define ischemic burden and a threshold for benefit of percutaneous vs medical management of CAD. Ischemia greater than 10% on myocardial perfusion imaging is associated with a high risk of major adverse cardiac events (MACE).9 Similar findings were noted in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy, which showed superior reduction in angina and MACE in patients with greater than 10% ischemia on myocardial perfusion imaging treated with PCI vs medical therapy.10 In the case of coronary CTO, ischemia greater than 12.5% is predictive of significant improvement in symptoms after intervention.11
PROGNOSIS AND DISEASE BURDEN
CTO is associated with adverse prognosis, implying the importance of incomplete revascularization. The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) trial used a scoring system to direct surgical vs percutaneous revascularization strategies in patients with complex or multivessel CAD. A post hoc analysis of the SYNTAX trial showed that incomplete revascularization was associated with significantly higher rates of 4-year mortality and MACE.12 This was likely from the ischemic burden remaining from incomplete revascularization. The presence of CTO was the strongest independent predictor of incomplete revascularization in the SYNTAX PCI arm. Similarly, the negative prognostic impact of having a CTO has been observed in a large population of patients followed prospectively after undergoing coronary angiography.13 Furthermore, the presence of CTO in a non-infarct-related artery at the time of ST-elevation myocardial infarction appears to be an independent predictor of death at 30 days, with a persistent negative prognostic impact lasting for up to 36 months of follow-up.14
CLINICAL BENEFITS OF CTO PCI
In patients with significant ischemic burden, CTO PCI has multiple clinical benefits. Symptomatic relief based on the Seattle Angina Questionnaire appears to be similar to that obtained with coronary artery bypass grafting (CABG) at 1-month follow up.15 Successful CTO PCI can have a positive impact on the risk of mortality in prospective13 and retrospective observational studies.16
CTO intervention may also have beneficial effects on left ventricular systolic function in patients with viable myocardium in the corresponding coronary territory.17 This improvement in systolic function appears to be sustained at 3 years of follow-up.18 Meta-analysis of observational data in symptomatic and ischemic patients who underwent successful CTO PCI shows reduced rates of all-cause mortality and MACE and a reduced need for subsequent CABG.19 This is in contrast to the frequently cited Occluded Artery Trial (OAT) trial, which showed no clinical benefit of PCI for a subacutely occluded infarct-related artery.20
EVIDENCE-BASED BENEFITS
Evidence of the merits of CTO PCI from randomized clinical trials is mixed. The only published study to date, the Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation (EXPLORE) trial, showed no difference in left ventricular systolic function 4 months after ST-elevation myocardial infarction in patients undergoing staged CTO PCI of a non-infarct-related artery vs optimal medical therapy.21 Two larger trials presented at scientific meetings in 2017 remain unpublished. One trial showed noninferiority of optimal medical therapy vs successful CTO PCI in reducing the composite end point of all-cause mortality, myocardial infarction, stroke, and repeat revascularization; the other trial showed significant improvement in quality of life measures using the Seattle Angina Questionnaire score and Canadian Cardiovascular Society angina classification in patients who underwent successful CTO PCI compared with medical management.
High-volume CTO PCI centers now report procedural success rates as high as 92.9%22 and a correlation between the CTO PCI volume and CTO PCI success rates.3 The dramatic improvement in success rates achieved by high-volume operators globally can be attributed to a combination of operator experience, improved technology, and widespread adoption of the hybrid algorithm, which has helped to improve efficiency and standardize treatment in CTO PCI based on angiographic criteria.23 CTO PCI remains a highly specialized procedure, unique from patent-vessel PCI and with little correlation between total PCI volume and CTO PCI success rate. Despite recent advances, CTO PCI success remains heavily dependent on operator expertises, with a steep and long learning curve. In addition, the unique technical aspects of CTO PCI such as a retrograde and subintimal guidewire tracking that have accelerated procedural success are associated with higher rates of MACE compared with traditional antegrade and intraluminal guidewire tracking.24,25 Therefore, CTO PCI requires unique considerations beyond standard PCI in terms of potential complications. Uncommon but potentially life-threatening complications such as donor artery thrombosis, collateral vessel trauma, gear entrapment, and radiation skin injury demand a specialized informed consent process for the patient.26
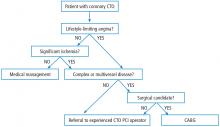
In light of incomplete evidence based on extensive observational data and limited randomized clinical trials, the decision to refer patients for CTO PCI requires a comprehensive clinical evaluation. We know from data derived from patients with patent but stenotic coronary arteries that physiologically rather than angiographically driven decisions to revascularize can produce superior clinical results. There is an ischemic burden threshold beyond which revascularization is superior to optimal medical therapy. In this context, we know that CTO is not benign and is associated with ischemic burden. Consequently, patients with symptoms related to CTO represent a subset of patients with incomplete revascularization.
CONCLUSION
Despite recent advances, CTO PCI procedures remain technically demanding, and success with a low complication rate is heavily dependent on operator expertise. Therefore, CTO PCI should be used judiciously in patients with angina refractory to optimal medical therapy. It is an important tool to be used in conjunction with non-CTO PCI, CABG, and optimal medical therapy to produce favorable outcomes in patients with CAD.
- De Bruyne B, Pijls NHJ, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224.
- Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 2015; 8:245–253.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Author/Task Force members; Windeker S, Kolh P, Alfonso R, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35:2541–2619.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv 2014; 83:9–16.
- Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J 2006; 27:2406–2412.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:E453–E458.
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107:2900–2907.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Safley DM, Koshy S, Grantham JA, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv 2011; 78:337–343.
- Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol 2013; 61:282–294.
- Råmunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv 2016; 9:1535–1544.
- Claessen BE, Dangas GD, Weisz G, et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J 2012; 33:768–775.
- Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes 2010; 3:284–290.
- Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on long-term outcome in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol 2013; 165:76–79.
- Baks T, van Geuns R-J, Duncker DJ, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol 2006; 47:721–725.
- Kirschbaum SW, Baks T, van den Ent M, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol 2008; 101:179–185.
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:95–107.
- Hochman JS, Lamas GA, Buller CE, et al; Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355:2395–2407.
- Henriques JP, Hoebers LP, Råmunddal T, et al; EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: The EXPLORE trial. J Am Coll Cardiol 2016; 68:1622–1632.
- Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv 2016; 9:1–9.
- Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv 2012; 5:367–379.
- Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv 2016; 9. pii: e003434. doi:10.1161/CIRCINTERVENTIONS.115.003434.
- Song L, Maehara A, Finn MT, et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc Interv 2017; 10:1011–1021.
- Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv 2013; 6:128–136.
In patients with stable coronary artery disease (CAD), the cornerstone of treatment is medical management to control symptoms such as angina and dyspnea on exertion. But in a select group of patients, percutaneous coronary intervention (PCI) is indicated in addition to medical management. Invasive and noninvasive hemodynamic assessments of coronary artery stenosis in conjunction with anatomic considerations play a role in decision-making and in advising patients on revascularization vs medical management. However, in the case of coronary artery chronic total occlusion (CTO), the decision-making process remains challenging due to limited evidence supporting clinical efficacy of CTO PCI, as well as practical considerations including lower success rates and higher complication rates in comparison with patent-vessel PCI.
CLINICAL VIGNETTE
A 42-year-old man, an avid runner with hyperlipidemia and a strong family history of premature CAD, presents with several months of declining exercise tolerance. His physical examination and electrocardiogram are unremarkable. Myocardial perfusion imaging shows stress-induced ischemia affecting about 20% of the inferolateral myocardium. He is then referred for coronary angiography.
- Panel A: Discrete, high-grade stenosis of the mid-right coronary artery
- Panel B: Diffuse, multivessel disease involving the distal right coronary artery (B1) and the proximal left circumflex coronary artery (B2)
- Panel C: Total occlusion of the proximal right coronary artery with extensive left-to-right collaterals.
Treatment based on angiographic findings
In panel A, there is little to debate. The patient is likely to benefit from percutaneous revascularization of the right coronary artery to treat symptoms.
In panels B1 and B2, there is abundant evidence that the hemodynamic assessment of stenosis is superior to a visual estimate in directing PCI.1,2 Hemodynamic assessments including fractional flow reserve (FFR) inform the risk-benefit analysis of percutaneous vs medical treatment of coronary stenosis. In the case of FFR, 0.8 represents an inflection point. The lower FFR values are below 0.8, the greater the benefit of PCI as opposed to medical therapy. Conversely, the greater FFR values are above 0.8, the greater the benefit of medical therapy as opposed to PCI.
However, in panel C, there is significant variability in the data supporting the best treatment strategy for symptomatic patients with CTO.
CORONARY CTO
Coronary CTO is defined as TIMI 0 flow for more than 3 months in an epicardial coronary artery. CTO is not uncommon, seen on 30% of routine coronary angiograms. In the United States, attempt rates of PCI for CTO remain low and have been static at around 12.4%, representing less than 5% of total PCI volume.3 In addition, success rates of CTO PCI are disappointingly low at 59% compared with success rates of patent-vessel PCI at 96%.3 The most frequently cited barriers to CTO PCI are incomplete evidence for efficacy and concerns about safety. Because of the ongoing controversy about the risks and benefits of CTO PCI, it remains a class IIa indication in current American and European practice guidelines.4,5 In addition, these procedures remain technically challenging, and thus variability in local expertise can influence the decision to manage patients medically or refer for CTO PCI.
Patients are often advised that CTO is benign. However, the myocardium affected by a CTO is ischemic. Collateral vessels do not provide adequate flow reserve. FFR data collected from CTOs that were successfully crossed and subsequently interrogated with a pressure wire prior to stenting show that the myocardium supplied by the reconstituted distal bed remains ischemic. This ischemic burden appears to be independent of the size and quality of collaterals.6,7 In addition, a moderate stenosis in a donor coronary artery supplying collateral vessels to a CTO may result in an ischemic FFR as a consequence of coronary “steal” from the donor artery to the collateral vessels. The ischemic FFR in the donor artery can be corrected by treating the recipient CTO vessel.8
Similar to FFR, noninvasive assessment using myocardial perfusion imaging can define ischemic burden and a threshold for benefit of percutaneous vs medical management of CAD. Ischemia greater than 10% on myocardial perfusion imaging is associated with a high risk of major adverse cardiac events (MACE).9 Similar findings were noted in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy, which showed superior reduction in angina and MACE in patients with greater than 10% ischemia on myocardial perfusion imaging treated with PCI vs medical therapy.10 In the case of coronary CTO, ischemia greater than 12.5% is predictive of significant improvement in symptoms after intervention.11
PROGNOSIS AND DISEASE BURDEN
CTO is associated with adverse prognosis, implying the importance of incomplete revascularization. The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) trial used a scoring system to direct surgical vs percutaneous revascularization strategies in patients with complex or multivessel CAD. A post hoc analysis of the SYNTAX trial showed that incomplete revascularization was associated with significantly higher rates of 4-year mortality and MACE.12 This was likely from the ischemic burden remaining from incomplete revascularization. The presence of CTO was the strongest independent predictor of incomplete revascularization in the SYNTAX PCI arm. Similarly, the negative prognostic impact of having a CTO has been observed in a large population of patients followed prospectively after undergoing coronary angiography.13 Furthermore, the presence of CTO in a non-infarct-related artery at the time of ST-elevation myocardial infarction appears to be an independent predictor of death at 30 days, with a persistent negative prognostic impact lasting for up to 36 months of follow-up.14
CLINICAL BENEFITS OF CTO PCI
In patients with significant ischemic burden, CTO PCI has multiple clinical benefits. Symptomatic relief based on the Seattle Angina Questionnaire appears to be similar to that obtained with coronary artery bypass grafting (CABG) at 1-month follow up.15 Successful CTO PCI can have a positive impact on the risk of mortality in prospective13 and retrospective observational studies.16
CTO intervention may also have beneficial effects on left ventricular systolic function in patients with viable myocardium in the corresponding coronary territory.17 This improvement in systolic function appears to be sustained at 3 years of follow-up.18 Meta-analysis of observational data in symptomatic and ischemic patients who underwent successful CTO PCI shows reduced rates of all-cause mortality and MACE and a reduced need for subsequent CABG.19 This is in contrast to the frequently cited Occluded Artery Trial (OAT) trial, which showed no clinical benefit of PCI for a subacutely occluded infarct-related artery.20
EVIDENCE-BASED BENEFITS
Evidence of the merits of CTO PCI from randomized clinical trials is mixed. The only published study to date, the Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation (EXPLORE) trial, showed no difference in left ventricular systolic function 4 months after ST-elevation myocardial infarction in patients undergoing staged CTO PCI of a non-infarct-related artery vs optimal medical therapy.21 Two larger trials presented at scientific meetings in 2017 remain unpublished. One trial showed noninferiority of optimal medical therapy vs successful CTO PCI in reducing the composite end point of all-cause mortality, myocardial infarction, stroke, and repeat revascularization; the other trial showed significant improvement in quality of life measures using the Seattle Angina Questionnaire score and Canadian Cardiovascular Society angina classification in patients who underwent successful CTO PCI compared with medical management.
High-volume CTO PCI centers now report procedural success rates as high as 92.9%22 and a correlation between the CTO PCI volume and CTO PCI success rates.3 The dramatic improvement in success rates achieved by high-volume operators globally can be attributed to a combination of operator experience, improved technology, and widespread adoption of the hybrid algorithm, which has helped to improve efficiency and standardize treatment in CTO PCI based on angiographic criteria.23 CTO PCI remains a highly specialized procedure, unique from patent-vessel PCI and with little correlation between total PCI volume and CTO PCI success rate. Despite recent advances, CTO PCI success remains heavily dependent on operator expertises, with a steep and long learning curve. In addition, the unique technical aspects of CTO PCI such as a retrograde and subintimal guidewire tracking that have accelerated procedural success are associated with higher rates of MACE compared with traditional antegrade and intraluminal guidewire tracking.24,25 Therefore, CTO PCI requires unique considerations beyond standard PCI in terms of potential complications. Uncommon but potentially life-threatening complications such as donor artery thrombosis, collateral vessel trauma, gear entrapment, and radiation skin injury demand a specialized informed consent process for the patient.26
In light of incomplete evidence based on extensive observational data and limited randomized clinical trials, the decision to refer patients for CTO PCI requires a comprehensive clinical evaluation. We know from data derived from patients with patent but stenotic coronary arteries that physiologically rather than angiographically driven decisions to revascularize can produce superior clinical results. There is an ischemic burden threshold beyond which revascularization is superior to optimal medical therapy. In this context, we know that CTO is not benign and is associated with ischemic burden. Consequently, patients with symptoms related to CTO represent a subset of patients with incomplete revascularization.
CONCLUSION
Despite recent advances, CTO PCI procedures remain technically demanding, and success with a low complication rate is heavily dependent on operator expertise. Therefore, CTO PCI should be used judiciously in patients with angina refractory to optimal medical therapy. It is an important tool to be used in conjunction with non-CTO PCI, CABG, and optimal medical therapy to produce favorable outcomes in patients with CAD.
In patients with stable coronary artery disease (CAD), the cornerstone of treatment is medical management to control symptoms such as angina and dyspnea on exertion. But in a select group of patients, percutaneous coronary intervention (PCI) is indicated in addition to medical management. Invasive and noninvasive hemodynamic assessments of coronary artery stenosis in conjunction with anatomic considerations play a role in decision-making and in advising patients on revascularization vs medical management. However, in the case of coronary artery chronic total occlusion (CTO), the decision-making process remains challenging due to limited evidence supporting clinical efficacy of CTO PCI, as well as practical considerations including lower success rates and higher complication rates in comparison with patent-vessel PCI.
CLINICAL VIGNETTE
A 42-year-old man, an avid runner with hyperlipidemia and a strong family history of premature CAD, presents with several months of declining exercise tolerance. His physical examination and electrocardiogram are unremarkable. Myocardial perfusion imaging shows stress-induced ischemia affecting about 20% of the inferolateral myocardium. He is then referred for coronary angiography.
- Panel A: Discrete, high-grade stenosis of the mid-right coronary artery
- Panel B: Diffuse, multivessel disease involving the distal right coronary artery (B1) and the proximal left circumflex coronary artery (B2)
- Panel C: Total occlusion of the proximal right coronary artery with extensive left-to-right collaterals.
Treatment based on angiographic findings
In panel A, there is little to debate. The patient is likely to benefit from percutaneous revascularization of the right coronary artery to treat symptoms.
In panels B1 and B2, there is abundant evidence that the hemodynamic assessment of stenosis is superior to a visual estimate in directing PCI.1,2 Hemodynamic assessments including fractional flow reserve (FFR) inform the risk-benefit analysis of percutaneous vs medical treatment of coronary stenosis. In the case of FFR, 0.8 represents an inflection point. The lower FFR values are below 0.8, the greater the benefit of PCI as opposed to medical therapy. Conversely, the greater FFR values are above 0.8, the greater the benefit of medical therapy as opposed to PCI.
However, in panel C, there is significant variability in the data supporting the best treatment strategy for symptomatic patients with CTO.
CORONARY CTO
Coronary CTO is defined as TIMI 0 flow for more than 3 months in an epicardial coronary artery. CTO is not uncommon, seen on 30% of routine coronary angiograms. In the United States, attempt rates of PCI for CTO remain low and have been static at around 12.4%, representing less than 5% of total PCI volume.3 In addition, success rates of CTO PCI are disappointingly low at 59% compared with success rates of patent-vessel PCI at 96%.3 The most frequently cited barriers to CTO PCI are incomplete evidence for efficacy and concerns about safety. Because of the ongoing controversy about the risks and benefits of CTO PCI, it remains a class IIa indication in current American and European practice guidelines.4,5 In addition, these procedures remain technically challenging, and thus variability in local expertise can influence the decision to manage patients medically or refer for CTO PCI.
Patients are often advised that CTO is benign. However, the myocardium affected by a CTO is ischemic. Collateral vessels do not provide adequate flow reserve. FFR data collected from CTOs that were successfully crossed and subsequently interrogated with a pressure wire prior to stenting show that the myocardium supplied by the reconstituted distal bed remains ischemic. This ischemic burden appears to be independent of the size and quality of collaterals.6,7 In addition, a moderate stenosis in a donor coronary artery supplying collateral vessels to a CTO may result in an ischemic FFR as a consequence of coronary “steal” from the donor artery to the collateral vessels. The ischemic FFR in the donor artery can be corrected by treating the recipient CTO vessel.8
Similar to FFR, noninvasive assessment using myocardial perfusion imaging can define ischemic burden and a threshold for benefit of percutaneous vs medical management of CAD. Ischemia greater than 10% on myocardial perfusion imaging is associated with a high risk of major adverse cardiac events (MACE).9 Similar findings were noted in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy, which showed superior reduction in angina and MACE in patients with greater than 10% ischemia on myocardial perfusion imaging treated with PCI vs medical therapy.10 In the case of coronary CTO, ischemia greater than 12.5% is predictive of significant improvement in symptoms after intervention.11
PROGNOSIS AND DISEASE BURDEN
CTO is associated with adverse prognosis, implying the importance of incomplete revascularization. The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) trial used a scoring system to direct surgical vs percutaneous revascularization strategies in patients with complex or multivessel CAD. A post hoc analysis of the SYNTAX trial showed that incomplete revascularization was associated with significantly higher rates of 4-year mortality and MACE.12 This was likely from the ischemic burden remaining from incomplete revascularization. The presence of CTO was the strongest independent predictor of incomplete revascularization in the SYNTAX PCI arm. Similarly, the negative prognostic impact of having a CTO has been observed in a large population of patients followed prospectively after undergoing coronary angiography.13 Furthermore, the presence of CTO in a non-infarct-related artery at the time of ST-elevation myocardial infarction appears to be an independent predictor of death at 30 days, with a persistent negative prognostic impact lasting for up to 36 months of follow-up.14
CLINICAL BENEFITS OF CTO PCI
In patients with significant ischemic burden, CTO PCI has multiple clinical benefits. Symptomatic relief based on the Seattle Angina Questionnaire appears to be similar to that obtained with coronary artery bypass grafting (CABG) at 1-month follow up.15 Successful CTO PCI can have a positive impact on the risk of mortality in prospective13 and retrospective observational studies.16
CTO intervention may also have beneficial effects on left ventricular systolic function in patients with viable myocardium in the corresponding coronary territory.17 This improvement in systolic function appears to be sustained at 3 years of follow-up.18 Meta-analysis of observational data in symptomatic and ischemic patients who underwent successful CTO PCI shows reduced rates of all-cause mortality and MACE and a reduced need for subsequent CABG.19 This is in contrast to the frequently cited Occluded Artery Trial (OAT) trial, which showed no clinical benefit of PCI for a subacutely occluded infarct-related artery.20
EVIDENCE-BASED BENEFITS
Evidence of the merits of CTO PCI from randomized clinical trials is mixed. The only published study to date, the Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation (EXPLORE) trial, showed no difference in left ventricular systolic function 4 months after ST-elevation myocardial infarction in patients undergoing staged CTO PCI of a non-infarct-related artery vs optimal medical therapy.21 Two larger trials presented at scientific meetings in 2017 remain unpublished. One trial showed noninferiority of optimal medical therapy vs successful CTO PCI in reducing the composite end point of all-cause mortality, myocardial infarction, stroke, and repeat revascularization; the other trial showed significant improvement in quality of life measures using the Seattle Angina Questionnaire score and Canadian Cardiovascular Society angina classification in patients who underwent successful CTO PCI compared with medical management.
High-volume CTO PCI centers now report procedural success rates as high as 92.9%22 and a correlation between the CTO PCI volume and CTO PCI success rates.3 The dramatic improvement in success rates achieved by high-volume operators globally can be attributed to a combination of operator experience, improved technology, and widespread adoption of the hybrid algorithm, which has helped to improve efficiency and standardize treatment in CTO PCI based on angiographic criteria.23 CTO PCI remains a highly specialized procedure, unique from patent-vessel PCI and with little correlation between total PCI volume and CTO PCI success rate. Despite recent advances, CTO PCI success remains heavily dependent on operator expertises, with a steep and long learning curve. In addition, the unique technical aspects of CTO PCI such as a retrograde and subintimal guidewire tracking that have accelerated procedural success are associated with higher rates of MACE compared with traditional antegrade and intraluminal guidewire tracking.24,25 Therefore, CTO PCI requires unique considerations beyond standard PCI in terms of potential complications. Uncommon but potentially life-threatening complications such as donor artery thrombosis, collateral vessel trauma, gear entrapment, and radiation skin injury demand a specialized informed consent process for the patient.26
In light of incomplete evidence based on extensive observational data and limited randomized clinical trials, the decision to refer patients for CTO PCI requires a comprehensive clinical evaluation. We know from data derived from patients with patent but stenotic coronary arteries that physiologically rather than angiographically driven decisions to revascularize can produce superior clinical results. There is an ischemic burden threshold beyond which revascularization is superior to optimal medical therapy. In this context, we know that CTO is not benign and is associated with ischemic burden. Consequently, patients with symptoms related to CTO represent a subset of patients with incomplete revascularization.
CONCLUSION
Despite recent advances, CTO PCI procedures remain technically demanding, and success with a low complication rate is heavily dependent on operator expertise. Therefore, CTO PCI should be used judiciously in patients with angina refractory to optimal medical therapy. It is an important tool to be used in conjunction with non-CTO PCI, CABG, and optimal medical therapy to produce favorable outcomes in patients with CAD.
- De Bruyne B, Pijls NHJ, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224.
- Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 2015; 8:245–253.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Author/Task Force members; Windeker S, Kolh P, Alfonso R, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35:2541–2619.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv 2014; 83:9–16.
- Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J 2006; 27:2406–2412.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:E453–E458.
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107:2900–2907.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Safley DM, Koshy S, Grantham JA, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv 2011; 78:337–343.
- Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol 2013; 61:282–294.
- Råmunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv 2016; 9:1535–1544.
- Claessen BE, Dangas GD, Weisz G, et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J 2012; 33:768–775.
- Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes 2010; 3:284–290.
- Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on long-term outcome in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol 2013; 165:76–79.
- Baks T, van Geuns R-J, Duncker DJ, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol 2006; 47:721–725.
- Kirschbaum SW, Baks T, van den Ent M, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol 2008; 101:179–185.
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:95–107.
- Hochman JS, Lamas GA, Buller CE, et al; Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355:2395–2407.
- Henriques JP, Hoebers LP, Råmunddal T, et al; EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: The EXPLORE trial. J Am Coll Cardiol 2016; 68:1622–1632.
- Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv 2016; 9:1–9.
- Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv 2012; 5:367–379.
- Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv 2016; 9. pii: e003434. doi:10.1161/CIRCINTERVENTIONS.115.003434.
- Song L, Maehara A, Finn MT, et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc Interv 2017; 10:1011–1021.
- Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv 2013; 6:128–136.
- De Bruyne B, Pijls NHJ, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224.
- Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 2015; 8:245–253.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Author/Task Force members; Windeker S, Kolh P, Alfonso R, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35:2541–2619.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv 2014; 83:9–16.
- Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J 2006; 27:2406–2412.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:E453–E458.
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107:2900–2907.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Safley DM, Koshy S, Grantham JA, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv 2011; 78:337–343.
- Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol 2013; 61:282–294.
- Råmunddal T, Hoebers LP, Henriques JP, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv 2016; 9:1535–1544.
- Claessen BE, Dangas GD, Weisz G, et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J 2012; 33:768–775.
- Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes 2010; 3:284–290.
- Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on long-term outcome in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol 2013; 165:76–79.
- Baks T, van Geuns R-J, Duncker DJ, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol 2006; 47:721–725.
- Kirschbaum SW, Baks T, van den Ent M, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol 2008; 101:179–185.
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv 2013; 82:95–107.
- Hochman JS, Lamas GA, Buller CE, et al; Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355:2395–2407.
- Henriques JP, Hoebers LP, Råmunddal T, et al; EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: The EXPLORE trial. J Am Coll Cardiol 2016; 68:1622–1632.
- Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv 2016; 9:1–9.
- Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv 2012; 5:367–379.
- Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv 2016; 9. pii: e003434. doi:10.1161/CIRCINTERVENTIONS.115.003434.
- Song L, Maehara A, Finn MT, et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc Interv 2017; 10:1011–1021.
- Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv 2013; 6:128–136.
KEY POINTS
- Coronary CTO is not benign and is associated with ischemic burden.
- There is a threshold of ischemic burden at which revascularization is superior to optimal medical therapy.
- Revascularization based on physiology rather than angiography can produce superior clinical results.
- CTO PCI procedures are technically demanding and heavily operator-dependent in order to achieve high success rates at an acceptably low complication rate.