User login
› Prescribe an anti-inflammatory drug whenever you initiate urate-lowering therapy (ULT). A
› Do not initiate ULT during an acute gout attack; if a patient on an established ULT regimen has an acute attack, however, therapy should not be stopped. C
› Increase the dosage of ULT to achieve a lower target if gout symptoms persist despite a serum urate level <6 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
From the 1960s to the ’90s, the prevalence of gout more than doubled among US residents.1 In the years since,1-5 gout has become increasingly prevalent worldwide. The key causes—an aging population, poor diet, widespread use of diuretics to treat cardiovascular disease, and comorbidities that promote hyperuricemia—have made the presentation of gout more complex and harder to manage, as well.4-6
In fact, gout is frequently poorly managed. Initiation and maintenance of urate-lowering therapy (ULT)—as well as monitoring of serum urate—is not done often enough, and there is significant variation in medications used to treat gout. As a result, recommended serum urate targets commonly remain unattained.7-9
We can do better. Enhanced understanding of risk factors for gout, augmented by recent research and the approval of 2 new pharmacologic agents (febuxostat in 200910 and pegloticase in 201011), led to the American College of Rheumatology (ACR)’s first edition of gout guidelines, published in 2012.12,13 The key components of gout management—patient education, lifestyle modifications, and pharmacologic therapy—are detailed in the text and tables that follow.
Understanding gout
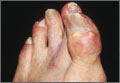
Gout is actually a heterogeneous spectrum of diseases. It is characterized by an elevated serum urate concentration, with recurrent attacks of acute arthritis associated with monosodium urate crystals in synovial fluid leukocytes, but may also include tophi— typically painless nodular deposits of monosodium urate crystals in tissues in and around the joints—interstitial renal disease, and uric acid nephrolithiasis.14 Symptoms occur when the excess uric acid, the result of inefficient excretion rather than overproduction,2,12,14,15 is deposited in restricted joint spaces.
Who’s at risk?
Risk factors include numerous cardiovascular and metabolic conditions, such as increased adiposity, hypertension, dyslipidemia, heart failure, insulin resistance, hyperglycemia, and renal disease.3,6,12 Older age, genetics, poor diet, alcohol consumption, and medications associated with hyperuricemia, such as loop and thiazide diuretics and low-dose acetylsalicylic acid, are risk factors, as well.
Defined as a serum urate level ≥6.8 mg/dL—the point at which urate becomes insoluble in extracellular fluids12,16,17—hyperuricemia is the most important modifiable risk factor for the development of gout. It can precipitate painful episodic attacks and complications such as chronic arthritis, urolithiasis, and tophi.12
What you’ll see
Patients often present with acute onset of pain and inflammation of a single joint, usually the first metatarsophalangeal. Other joints and soft tissues that may be involved to a lesser extent include (in order of frequency) the insteps, ankles, heels, knees, fingers, and elbows.14 Polyarticular attacks are an atypical manifestation and are sometimes confused with rheumatoid arthritis or osteoarthritis, particularly in the elderly.
Clinical evaluation should include a history of symptom severity, disease burden, and comorbidities, and a thorough physical examination focused on findings such as tophi and acute and chronic synovitis.12 Imaging studies are not recommended for the evaluation of gout because therapy is guided by symptoms.14
Asymptomatic hyperuricemia alone does not establish a diagnosis of gout, and there is no evidence to support ULT for isolated hyperuricemia. However, advice regarding lifestyle modifications and treatment of associated comorbidities may be warranted for such patients.18
How best to manage gout
Optimal gout management encompasses nonpharmacologic therapy, symptom management of acute attacks, and combination anti-inflammatory and ULT prophylaxis for patients with chronic gout.12,13 It is important to work with patients to track and document both the number and the severity of acute attacks occurring over a 12-month period so that those who qualify for ULT can begin it without delay.12 It is important to discuss treatment objectives and management of comorbidities, as well.
Review the medications the patient is taking, and consider eliminating prescription drugs associated with hyperuricemia if the risks outweigh the benefits.19-21 In many cases, however, lifestyle modification—ie, eating a heart-healthy diet, exercising regularly, and losing weight—may do more to prevent gout attacks and manage complications than stopping medications that provide cardioprotection.6 The ACR divides food and beverages into 3 simple categories—avoid, limit, or encourage (TABLE 1.)12
Responding to an acute attack
Whenever possible, initiate pharmacologic therapy within 24 hours of symptom onset, because this has been associated with decreased pain and shorter duration of an acute attack.8,13 The choice of drug should be guided by the severity of the attack, as determined by both a pain score on a visual analog scale (VAS) and the number of affected joints; patient preference, prior response, and associated comorbidities are also important considerations (TABLE 28,13,14). When medications are prescribed for acute attacks or chronic gout, a discussion of adverse effects, drug interactions, contraindications, cost, and the importance of adherence is needed, as well.
For mild to moderate pain (≤6 out of 10 on a VAS) involving a few small joints or one or 2 large joints, monotherapy with a nonsteroidal anti-inflammatory drug (NSAID), a corticosteroid, or colchicine is recommended. For severe pain (>6 out of 10) and/or polyarticular involvement (≥4 joints in more than one region of the body), combination therapy is recommended (eg, colchicine and either an NSAID or a corticosteroid).13 Prednisone, methylprednisolone, and adrenocorticotropic hormone are options for patients who are NPO. Acute gout therapy should be continued until the attack resolves, which can range from 5 to 14 days.13
Colchicine considerations. The dose of colchicine recommended by the ACR for an acute gout attack (1.2 mg loading dose, followed by 0.6 mg one hour later, then followed after 12 hours, as needed, by up to 0.6 mg once or twice a day) is substantially lower than the dosing schedule used historically (1.0 mg loading dose, followed by 0.5 mg every 2-3 hours). Higher doses have not proven to be more effective, however, and typically led to gastrointestinal toxicity, causing patients to stop taking the drug before acute symptoms resolved.8,13,22
Keep in mind, too, that colchicine therapy should not be initiated more than 36 hours after symptom onset, as therapy is less effective beyond this time frame.8,13 In addition, concurrent use with P-glycoprotein and CYP3A4 inhibitors—eg, clarithromycin and erythromycin and some antifungals, antiretrovirals, calcium-channel blockers, immunosuppressants, and statins—may increase the risk of colchicine toxicity and should be avoided.
Treating chronic gout
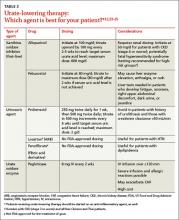
Management of recurrent or progressive gout is aimed at reducing and maintaining serum urate levels <6.0 mg/dL, using ULT (TABLE 312,23-25) combined with anti-inflammatory prophylaxis to reduce the frequency of gout flares and the size and number of tophi.12,23 Patients who meet one or more of the following criteria qualify for ULT:
- the presence of tophi
- ≥2 acute attacks per year
- chronic kidney disease (CKD) stages 2 through 5
- a history of urolithiasis.12
Both ULT and anti-inflammatory therapy should be started after an acute gout attack resolves, but patients already on prophylactic therapy should continue the regimen both during and after acute attacks to avoid more frequent exacerbations.9,12 If gout symptoms persist despite a serum urate level of <6.0 mg/dL, increase the dose of ULT to achieve a target of <5 mg/dL to reduce the frequency of flares and the size and number of tophi.12,26
Allopurinol, a xanthine oxidase inhibitor, is typically used as first-line ULT due to efficacy and low cost.13 Febuxostat, also a xanthine oxidase inhibitor, is an additional first-line option, although the US Food and Drug Administration issued a warning based on postmarketing reports of hepatic failure.25 In the case of a xanthine oxidase allergy or intolerance, probenecid may be used as an alternative first-line therapy. First-line agents for anti-inflammatory prophylaxis include low-dose colchicine (0.6 mg once or twice daily) and low-dose NSAIDs. Oral corticosteroids (<10 mg/d) are considered second-line therapy.13
Allopurinol hypersensitivity. Although allopurinol is generally well tolerated, about 2% of patients develop a mild rash and up to 5% of patients stop taking it because of an adverse effect.25 More importantly, allopurinol hypersensitivity syndrome (AHS) is rare but potentially fatal; in the United States, it is estimated that one in every 1000 patients treated with allopurinol will develop AHS.12,27
AHS is characterized by a rash (eg, Stevens-Johnson syndrome or toxic epidermal necrolysis), eosinophilia, leukocytosis, fever, hepatitis, and renal failure.12,25 There is no cure; the mainstay of treatment is early diagnosis, withdrawal of allopurinol, and supportive care.25 Because of the high mortality rate (20%-25%),12,27 genetic screening for allele HLA-B*5801 prior to starting allopurinol therapy is recommended for patients in high-risk groups: Koreans with CKD (stage 3 or worse) and all Han Chinese and Thai patients, regardless of kidney function.12 Alternative therapies should be used for patients who test positive for the allele.
Duration of therapy
Pharmacologic treatment of an acute gout attack should continue until the attack resolves, which can range from 5 to 14 days. The duration of treatment for chronic gout is far longer.
Anti-inflammatory prophylaxis should continue for whichever is greater: 3 months after the target serum urate level is achieved for patients with no evidence of tophi; or 6 months after the target serum urate level is achieved and previously detected tophi have resolved.13
ULT should continue indefinitely,12 with monitoring of serum urate levels every 2 to 5 weeks until the target is achieved and every 6 months thereafter.
Not responding to therapy? Consider nonadherence, refractory gout
If a patient is not responding as expected, consider whether he or she is taking the medication as prescribed. Gout therapy has one of the lowest adherence rates of any chronic disorder.7,8,12,28-30 Studies have found that less than half of patients started on ULT take their medication as prescribed for the entire first year of therapy.9,28
Evidence suggests that nonadherence is especially likely among younger and healthier individuals, possibly because they have little experience managing chronic conditions or needing ongoing care.28-30 Such patients may also be unsure of when and how to take their medication. To promote adherence, physicians should schedule more frequent follow-up appointments after initiating ULT to assess management of the disease and stress the importance of following the medication regimen as prescribed.9,28
Not all patients who don’t respond to ULT are nonadherent, of course. Some have refractory gout. If uric acid levels do not reach the goal of <6 mg/dL (or <5 mg/dL) at the maximum dose of a first-line xanthine oxidase inhibitor, add a uricosuric agent such as probenecid, fenofibrate, or losartan.12
Pegloticase, a pegylated recombinant form of urate oxidase enzyme that converts uric acid to allantoin31 (a water-soluble metabolite of uric acid), is a possible therapeutic option for patients who do not achieve adequate serum urate levels and continue to have symptoms of gout.12 Candidates for pegloticase therapy, which is administered intravenously, include adult patients with gout refractory to conventional ULT or excessive uric acid accumulation due to chemotherapy and those with contraindications to conventional ULT.
Pegloticase is associated with anaphylactic and infusion reactions, requires extensive monitoring, and costs thousands of dollars per month, however. Thus, it is important to carefully evaluate the extent of disease burden (ie, gout symptoms and effect on quality of life) and determine whether the patient has taken ULT and uricosuric drugs as prescribed before considering this option. Pegloticase requires the same anti-inflammatory prophylaxis as other forms of ULT, but there is no consensus on the duration of use.12
CORRESPONDENCE
Tatum Mead, PharmD, University of Missouri-Kansas City School of Pharmacy, Health Sciences Building, Room 2243, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
1. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Brook RA, Forsythe A, Smeeding JE, et al. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813-2821.
3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
4. Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31: 1582-1587.
5. Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3:443-449.
6. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22: 165-172.
7. Dalbeth N, Lindsay K. The patient’s experience of gout: new insights to optimize management. Curr Rheumatol Rep. 2012;14:173-178.
8. Edwards NL. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr Rheumatol Rep. 2011;13:154-159.
9. Singh JA, Hodges JS, Asch SM. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis. 2009;68: 1265-1270.
10. Drugs.com. FDA approves Uloric (febuxostat) for the chronic management of hyperuricemia in patients with gout [press release]. February 13, 2009. Drugs.com Web site. Available at: http://www.drugs.com/newdrugs/fda-approves-uloric-febuxostat-chronic-management-hyperuricemia-patients-gout-1266.html. Accessed October 29, 2014.
11. US Food and Drug Administration. FDA approves new drug for gout [press release]. September 14, 2010. US Food and Drug Administration Web site. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225810.htm. Accessed October 29, 2014.
12. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
13. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
14. Fravel MA, Ernst ME, Clark EC. Gout and hyperuricemia. In: DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw Hill; 2014: 1505-1523.
15. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-452.
16. Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189-192. 17. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
18. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
19. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
20. McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64:121-129.
21. Caspi D, Lubart E, Graff E, et al. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43:103-108.
22. Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060-1068.
23. Wortmann RL, Macdonald PA, Hunt B, et al. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32:2386-2397.
24. US Food and Drug Administration. Uloric (febuxostat tablets). US Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm243770.htm. Accessed October 29, 2014.
25. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
26. Becker MA, Schumacher HR, Benjamin KL, et al; Gout National Study Group. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041-1048.
27. Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad Dermatol. 1979;1:365-374.
28. Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46.
29. Reach G. Treatment adherence in patients with gout. Joint Bone Spine. 2011;78:456-459.
30. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437-443.
31. Lexicomp. News from the world of pharmacology. Lexicomp Web site. Available at: https://www.lexi.com/individuals/pharmacists/newsletters.jsp?id=october_10. Accessed January 13, 2014.
› Prescribe an anti-inflammatory drug whenever you initiate urate-lowering therapy (ULT). A
› Do not initiate ULT during an acute gout attack; if a patient on an established ULT regimen has an acute attack, however, therapy should not be stopped. C
› Increase the dosage of ULT to achieve a lower target if gout symptoms persist despite a serum urate level <6 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
From the 1960s to the ’90s, the prevalence of gout more than doubled among US residents.1 In the years since,1-5 gout has become increasingly prevalent worldwide. The key causes—an aging population, poor diet, widespread use of diuretics to treat cardiovascular disease, and comorbidities that promote hyperuricemia—have made the presentation of gout more complex and harder to manage, as well.4-6
In fact, gout is frequently poorly managed. Initiation and maintenance of urate-lowering therapy (ULT)—as well as monitoring of serum urate—is not done often enough, and there is significant variation in medications used to treat gout. As a result, recommended serum urate targets commonly remain unattained.7-9
We can do better. Enhanced understanding of risk factors for gout, augmented by recent research and the approval of 2 new pharmacologic agents (febuxostat in 200910 and pegloticase in 201011), led to the American College of Rheumatology (ACR)’s first edition of gout guidelines, published in 2012.12,13 The key components of gout management—patient education, lifestyle modifications, and pharmacologic therapy—are detailed in the text and tables that follow.
Understanding gout
Gout is actually a heterogeneous spectrum of diseases. It is characterized by an elevated serum urate concentration, with recurrent attacks of acute arthritis associated with monosodium urate crystals in synovial fluid leukocytes, but may also include tophi— typically painless nodular deposits of monosodium urate crystals in tissues in and around the joints—interstitial renal disease, and uric acid nephrolithiasis.14 Symptoms occur when the excess uric acid, the result of inefficient excretion rather than overproduction,2,12,14,15 is deposited in restricted joint spaces.
Who’s at risk?
Risk factors include numerous cardiovascular and metabolic conditions, such as increased adiposity, hypertension, dyslipidemia, heart failure, insulin resistance, hyperglycemia, and renal disease.3,6,12 Older age, genetics, poor diet, alcohol consumption, and medications associated with hyperuricemia, such as loop and thiazide diuretics and low-dose acetylsalicylic acid, are risk factors, as well.
Defined as a serum urate level ≥6.8 mg/dL—the point at which urate becomes insoluble in extracellular fluids12,16,17—hyperuricemia is the most important modifiable risk factor for the development of gout. It can precipitate painful episodic attacks and complications such as chronic arthritis, urolithiasis, and tophi.12
What you’ll see
Patients often present with acute onset of pain and inflammation of a single joint, usually the first metatarsophalangeal. Other joints and soft tissues that may be involved to a lesser extent include (in order of frequency) the insteps, ankles, heels, knees, fingers, and elbows.14 Polyarticular attacks are an atypical manifestation and are sometimes confused with rheumatoid arthritis or osteoarthritis, particularly in the elderly.
Clinical evaluation should include a history of symptom severity, disease burden, and comorbidities, and a thorough physical examination focused on findings such as tophi and acute and chronic synovitis.12 Imaging studies are not recommended for the evaluation of gout because therapy is guided by symptoms.14
Asymptomatic hyperuricemia alone does not establish a diagnosis of gout, and there is no evidence to support ULT for isolated hyperuricemia. However, advice regarding lifestyle modifications and treatment of associated comorbidities may be warranted for such patients.18
How best to manage gout
Optimal gout management encompasses nonpharmacologic therapy, symptom management of acute attacks, and combination anti-inflammatory and ULT prophylaxis for patients with chronic gout.12,13 It is important to work with patients to track and document both the number and the severity of acute attacks occurring over a 12-month period so that those who qualify for ULT can begin it without delay.12 It is important to discuss treatment objectives and management of comorbidities, as well.
Review the medications the patient is taking, and consider eliminating prescription drugs associated with hyperuricemia if the risks outweigh the benefits.19-21 In many cases, however, lifestyle modification—ie, eating a heart-healthy diet, exercising regularly, and losing weight—may do more to prevent gout attacks and manage complications than stopping medications that provide cardioprotection.6 The ACR divides food and beverages into 3 simple categories—avoid, limit, or encourage (TABLE 1.)12
Responding to an acute attack
Whenever possible, initiate pharmacologic therapy within 24 hours of symptom onset, because this has been associated with decreased pain and shorter duration of an acute attack.8,13 The choice of drug should be guided by the severity of the attack, as determined by both a pain score on a visual analog scale (VAS) and the number of affected joints; patient preference, prior response, and associated comorbidities are also important considerations (TABLE 28,13,14). When medications are prescribed for acute attacks or chronic gout, a discussion of adverse effects, drug interactions, contraindications, cost, and the importance of adherence is needed, as well.
For mild to moderate pain (≤6 out of 10 on a VAS) involving a few small joints or one or 2 large joints, monotherapy with a nonsteroidal anti-inflammatory drug (NSAID), a corticosteroid, or colchicine is recommended. For severe pain (>6 out of 10) and/or polyarticular involvement (≥4 joints in more than one region of the body), combination therapy is recommended (eg, colchicine and either an NSAID or a corticosteroid).13 Prednisone, methylprednisolone, and adrenocorticotropic hormone are options for patients who are NPO. Acute gout therapy should be continued until the attack resolves, which can range from 5 to 14 days.13
Colchicine considerations. The dose of colchicine recommended by the ACR for an acute gout attack (1.2 mg loading dose, followed by 0.6 mg one hour later, then followed after 12 hours, as needed, by up to 0.6 mg once or twice a day) is substantially lower than the dosing schedule used historically (1.0 mg loading dose, followed by 0.5 mg every 2-3 hours). Higher doses have not proven to be more effective, however, and typically led to gastrointestinal toxicity, causing patients to stop taking the drug before acute symptoms resolved.8,13,22
Keep in mind, too, that colchicine therapy should not be initiated more than 36 hours after symptom onset, as therapy is less effective beyond this time frame.8,13 In addition, concurrent use with P-glycoprotein and CYP3A4 inhibitors—eg, clarithromycin and erythromycin and some antifungals, antiretrovirals, calcium-channel blockers, immunosuppressants, and statins—may increase the risk of colchicine toxicity and should be avoided.
Treating chronic gout
Management of recurrent or progressive gout is aimed at reducing and maintaining serum urate levels <6.0 mg/dL, using ULT (TABLE 312,23-25) combined with anti-inflammatory prophylaxis to reduce the frequency of gout flares and the size and number of tophi.12,23 Patients who meet one or more of the following criteria qualify for ULT:
- the presence of tophi
- ≥2 acute attacks per year
- chronic kidney disease (CKD) stages 2 through 5
- a history of urolithiasis.12
Both ULT and anti-inflammatory therapy should be started after an acute gout attack resolves, but patients already on prophylactic therapy should continue the regimen both during and after acute attacks to avoid more frequent exacerbations.9,12 If gout symptoms persist despite a serum urate level of <6.0 mg/dL, increase the dose of ULT to achieve a target of <5 mg/dL to reduce the frequency of flares and the size and number of tophi.12,26
Allopurinol, a xanthine oxidase inhibitor, is typically used as first-line ULT due to efficacy and low cost.13 Febuxostat, also a xanthine oxidase inhibitor, is an additional first-line option, although the US Food and Drug Administration issued a warning based on postmarketing reports of hepatic failure.25 In the case of a xanthine oxidase allergy or intolerance, probenecid may be used as an alternative first-line therapy. First-line agents for anti-inflammatory prophylaxis include low-dose colchicine (0.6 mg once or twice daily) and low-dose NSAIDs. Oral corticosteroids (<10 mg/d) are considered second-line therapy.13
Allopurinol hypersensitivity. Although allopurinol is generally well tolerated, about 2% of patients develop a mild rash and up to 5% of patients stop taking it because of an adverse effect.25 More importantly, allopurinol hypersensitivity syndrome (AHS) is rare but potentially fatal; in the United States, it is estimated that one in every 1000 patients treated with allopurinol will develop AHS.12,27
AHS is characterized by a rash (eg, Stevens-Johnson syndrome or toxic epidermal necrolysis), eosinophilia, leukocytosis, fever, hepatitis, and renal failure.12,25 There is no cure; the mainstay of treatment is early diagnosis, withdrawal of allopurinol, and supportive care.25 Because of the high mortality rate (20%-25%),12,27 genetic screening for allele HLA-B*5801 prior to starting allopurinol therapy is recommended for patients in high-risk groups: Koreans with CKD (stage 3 or worse) and all Han Chinese and Thai patients, regardless of kidney function.12 Alternative therapies should be used for patients who test positive for the allele.
Duration of therapy
Pharmacologic treatment of an acute gout attack should continue until the attack resolves, which can range from 5 to 14 days. The duration of treatment for chronic gout is far longer.
Anti-inflammatory prophylaxis should continue for whichever is greater: 3 months after the target serum urate level is achieved for patients with no evidence of tophi; or 6 months after the target serum urate level is achieved and previously detected tophi have resolved.13
ULT should continue indefinitely,12 with monitoring of serum urate levels every 2 to 5 weeks until the target is achieved and every 6 months thereafter.
Not responding to therapy? Consider nonadherence, refractory gout
If a patient is not responding as expected, consider whether he or she is taking the medication as prescribed. Gout therapy has one of the lowest adherence rates of any chronic disorder.7,8,12,28-30 Studies have found that less than half of patients started on ULT take their medication as prescribed for the entire first year of therapy.9,28
Evidence suggests that nonadherence is especially likely among younger and healthier individuals, possibly because they have little experience managing chronic conditions or needing ongoing care.28-30 Such patients may also be unsure of when and how to take their medication. To promote adherence, physicians should schedule more frequent follow-up appointments after initiating ULT to assess management of the disease and stress the importance of following the medication regimen as prescribed.9,28
Not all patients who don’t respond to ULT are nonadherent, of course. Some have refractory gout. If uric acid levels do not reach the goal of <6 mg/dL (or <5 mg/dL) at the maximum dose of a first-line xanthine oxidase inhibitor, add a uricosuric agent such as probenecid, fenofibrate, or losartan.12
Pegloticase, a pegylated recombinant form of urate oxidase enzyme that converts uric acid to allantoin31 (a water-soluble metabolite of uric acid), is a possible therapeutic option for patients who do not achieve adequate serum urate levels and continue to have symptoms of gout.12 Candidates for pegloticase therapy, which is administered intravenously, include adult patients with gout refractory to conventional ULT or excessive uric acid accumulation due to chemotherapy and those with contraindications to conventional ULT.
Pegloticase is associated with anaphylactic and infusion reactions, requires extensive monitoring, and costs thousands of dollars per month, however. Thus, it is important to carefully evaluate the extent of disease burden (ie, gout symptoms and effect on quality of life) and determine whether the patient has taken ULT and uricosuric drugs as prescribed before considering this option. Pegloticase requires the same anti-inflammatory prophylaxis as other forms of ULT, but there is no consensus on the duration of use.12
CORRESPONDENCE
Tatum Mead, PharmD, University of Missouri-Kansas City School of Pharmacy, Health Sciences Building, Room 2243, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
› Prescribe an anti-inflammatory drug whenever you initiate urate-lowering therapy (ULT). A
› Do not initiate ULT during an acute gout attack; if a patient on an established ULT regimen has an acute attack, however, therapy should not be stopped. C
› Increase the dosage of ULT to achieve a lower target if gout symptoms persist despite a serum urate level <6 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
From the 1960s to the ’90s, the prevalence of gout more than doubled among US residents.1 In the years since,1-5 gout has become increasingly prevalent worldwide. The key causes—an aging population, poor diet, widespread use of diuretics to treat cardiovascular disease, and comorbidities that promote hyperuricemia—have made the presentation of gout more complex and harder to manage, as well.4-6
In fact, gout is frequently poorly managed. Initiation and maintenance of urate-lowering therapy (ULT)—as well as monitoring of serum urate—is not done often enough, and there is significant variation in medications used to treat gout. As a result, recommended serum urate targets commonly remain unattained.7-9
We can do better. Enhanced understanding of risk factors for gout, augmented by recent research and the approval of 2 new pharmacologic agents (febuxostat in 200910 and pegloticase in 201011), led to the American College of Rheumatology (ACR)’s first edition of gout guidelines, published in 2012.12,13 The key components of gout management—patient education, lifestyle modifications, and pharmacologic therapy—are detailed in the text and tables that follow.
Understanding gout
Gout is actually a heterogeneous spectrum of diseases. It is characterized by an elevated serum urate concentration, with recurrent attacks of acute arthritis associated with monosodium urate crystals in synovial fluid leukocytes, but may also include tophi— typically painless nodular deposits of monosodium urate crystals in tissues in and around the joints—interstitial renal disease, and uric acid nephrolithiasis.14 Symptoms occur when the excess uric acid, the result of inefficient excretion rather than overproduction,2,12,14,15 is deposited in restricted joint spaces.
Who’s at risk?
Risk factors include numerous cardiovascular and metabolic conditions, such as increased adiposity, hypertension, dyslipidemia, heart failure, insulin resistance, hyperglycemia, and renal disease.3,6,12 Older age, genetics, poor diet, alcohol consumption, and medications associated with hyperuricemia, such as loop and thiazide diuretics and low-dose acetylsalicylic acid, are risk factors, as well.
Defined as a serum urate level ≥6.8 mg/dL—the point at which urate becomes insoluble in extracellular fluids12,16,17—hyperuricemia is the most important modifiable risk factor for the development of gout. It can precipitate painful episodic attacks and complications such as chronic arthritis, urolithiasis, and tophi.12
What you’ll see
Patients often present with acute onset of pain and inflammation of a single joint, usually the first metatarsophalangeal. Other joints and soft tissues that may be involved to a lesser extent include (in order of frequency) the insteps, ankles, heels, knees, fingers, and elbows.14 Polyarticular attacks are an atypical manifestation and are sometimes confused with rheumatoid arthritis or osteoarthritis, particularly in the elderly.
Clinical evaluation should include a history of symptom severity, disease burden, and comorbidities, and a thorough physical examination focused on findings such as tophi and acute and chronic synovitis.12 Imaging studies are not recommended for the evaluation of gout because therapy is guided by symptoms.14
Asymptomatic hyperuricemia alone does not establish a diagnosis of gout, and there is no evidence to support ULT for isolated hyperuricemia. However, advice regarding lifestyle modifications and treatment of associated comorbidities may be warranted for such patients.18
How best to manage gout
Optimal gout management encompasses nonpharmacologic therapy, symptom management of acute attacks, and combination anti-inflammatory and ULT prophylaxis for patients with chronic gout.12,13 It is important to work with patients to track and document both the number and the severity of acute attacks occurring over a 12-month period so that those who qualify for ULT can begin it without delay.12 It is important to discuss treatment objectives and management of comorbidities, as well.
Review the medications the patient is taking, and consider eliminating prescription drugs associated with hyperuricemia if the risks outweigh the benefits.19-21 In many cases, however, lifestyle modification—ie, eating a heart-healthy diet, exercising regularly, and losing weight—may do more to prevent gout attacks and manage complications than stopping medications that provide cardioprotection.6 The ACR divides food and beverages into 3 simple categories—avoid, limit, or encourage (TABLE 1.)12
Responding to an acute attack
Whenever possible, initiate pharmacologic therapy within 24 hours of symptom onset, because this has been associated with decreased pain and shorter duration of an acute attack.8,13 The choice of drug should be guided by the severity of the attack, as determined by both a pain score on a visual analog scale (VAS) and the number of affected joints; patient preference, prior response, and associated comorbidities are also important considerations (TABLE 28,13,14). When medications are prescribed for acute attacks or chronic gout, a discussion of adverse effects, drug interactions, contraindications, cost, and the importance of adherence is needed, as well.
For mild to moderate pain (≤6 out of 10 on a VAS) involving a few small joints or one or 2 large joints, monotherapy with a nonsteroidal anti-inflammatory drug (NSAID), a corticosteroid, or colchicine is recommended. For severe pain (>6 out of 10) and/or polyarticular involvement (≥4 joints in more than one region of the body), combination therapy is recommended (eg, colchicine and either an NSAID or a corticosteroid).13 Prednisone, methylprednisolone, and adrenocorticotropic hormone are options for patients who are NPO. Acute gout therapy should be continued until the attack resolves, which can range from 5 to 14 days.13
Colchicine considerations. The dose of colchicine recommended by the ACR for an acute gout attack (1.2 mg loading dose, followed by 0.6 mg one hour later, then followed after 12 hours, as needed, by up to 0.6 mg once or twice a day) is substantially lower than the dosing schedule used historically (1.0 mg loading dose, followed by 0.5 mg every 2-3 hours). Higher doses have not proven to be more effective, however, and typically led to gastrointestinal toxicity, causing patients to stop taking the drug before acute symptoms resolved.8,13,22
Keep in mind, too, that colchicine therapy should not be initiated more than 36 hours after symptom onset, as therapy is less effective beyond this time frame.8,13 In addition, concurrent use with P-glycoprotein and CYP3A4 inhibitors—eg, clarithromycin and erythromycin and some antifungals, antiretrovirals, calcium-channel blockers, immunosuppressants, and statins—may increase the risk of colchicine toxicity and should be avoided.
Treating chronic gout
Management of recurrent or progressive gout is aimed at reducing and maintaining serum urate levels <6.0 mg/dL, using ULT (TABLE 312,23-25) combined with anti-inflammatory prophylaxis to reduce the frequency of gout flares and the size and number of tophi.12,23 Patients who meet one or more of the following criteria qualify for ULT:
- the presence of tophi
- ≥2 acute attacks per year
- chronic kidney disease (CKD) stages 2 through 5
- a history of urolithiasis.12
Both ULT and anti-inflammatory therapy should be started after an acute gout attack resolves, but patients already on prophylactic therapy should continue the regimen both during and after acute attacks to avoid more frequent exacerbations.9,12 If gout symptoms persist despite a serum urate level of <6.0 mg/dL, increase the dose of ULT to achieve a target of <5 mg/dL to reduce the frequency of flares and the size and number of tophi.12,26
Allopurinol, a xanthine oxidase inhibitor, is typically used as first-line ULT due to efficacy and low cost.13 Febuxostat, also a xanthine oxidase inhibitor, is an additional first-line option, although the US Food and Drug Administration issued a warning based on postmarketing reports of hepatic failure.25 In the case of a xanthine oxidase allergy or intolerance, probenecid may be used as an alternative first-line therapy. First-line agents for anti-inflammatory prophylaxis include low-dose colchicine (0.6 mg once or twice daily) and low-dose NSAIDs. Oral corticosteroids (<10 mg/d) are considered second-line therapy.13
Allopurinol hypersensitivity. Although allopurinol is generally well tolerated, about 2% of patients develop a mild rash and up to 5% of patients stop taking it because of an adverse effect.25 More importantly, allopurinol hypersensitivity syndrome (AHS) is rare but potentially fatal; in the United States, it is estimated that one in every 1000 patients treated with allopurinol will develop AHS.12,27
AHS is characterized by a rash (eg, Stevens-Johnson syndrome or toxic epidermal necrolysis), eosinophilia, leukocytosis, fever, hepatitis, and renal failure.12,25 There is no cure; the mainstay of treatment is early diagnosis, withdrawal of allopurinol, and supportive care.25 Because of the high mortality rate (20%-25%),12,27 genetic screening for allele HLA-B*5801 prior to starting allopurinol therapy is recommended for patients in high-risk groups: Koreans with CKD (stage 3 or worse) and all Han Chinese and Thai patients, regardless of kidney function.12 Alternative therapies should be used for patients who test positive for the allele.
Duration of therapy
Pharmacologic treatment of an acute gout attack should continue until the attack resolves, which can range from 5 to 14 days. The duration of treatment for chronic gout is far longer.
Anti-inflammatory prophylaxis should continue for whichever is greater: 3 months after the target serum urate level is achieved for patients with no evidence of tophi; or 6 months after the target serum urate level is achieved and previously detected tophi have resolved.13
ULT should continue indefinitely,12 with monitoring of serum urate levels every 2 to 5 weeks until the target is achieved and every 6 months thereafter.
Not responding to therapy? Consider nonadherence, refractory gout
If a patient is not responding as expected, consider whether he or she is taking the medication as prescribed. Gout therapy has one of the lowest adherence rates of any chronic disorder.7,8,12,28-30 Studies have found that less than half of patients started on ULT take their medication as prescribed for the entire first year of therapy.9,28
Evidence suggests that nonadherence is especially likely among younger and healthier individuals, possibly because they have little experience managing chronic conditions or needing ongoing care.28-30 Such patients may also be unsure of when and how to take their medication. To promote adherence, physicians should schedule more frequent follow-up appointments after initiating ULT to assess management of the disease and stress the importance of following the medication regimen as prescribed.9,28
Not all patients who don’t respond to ULT are nonadherent, of course. Some have refractory gout. If uric acid levels do not reach the goal of <6 mg/dL (or <5 mg/dL) at the maximum dose of a first-line xanthine oxidase inhibitor, add a uricosuric agent such as probenecid, fenofibrate, or losartan.12
Pegloticase, a pegylated recombinant form of urate oxidase enzyme that converts uric acid to allantoin31 (a water-soluble metabolite of uric acid), is a possible therapeutic option for patients who do not achieve adequate serum urate levels and continue to have symptoms of gout.12 Candidates for pegloticase therapy, which is administered intravenously, include adult patients with gout refractory to conventional ULT or excessive uric acid accumulation due to chemotherapy and those with contraindications to conventional ULT.
Pegloticase is associated with anaphylactic and infusion reactions, requires extensive monitoring, and costs thousands of dollars per month, however. Thus, it is important to carefully evaluate the extent of disease burden (ie, gout symptoms and effect on quality of life) and determine whether the patient has taken ULT and uricosuric drugs as prescribed before considering this option. Pegloticase requires the same anti-inflammatory prophylaxis as other forms of ULT, but there is no consensus on the duration of use.12
CORRESPONDENCE
Tatum Mead, PharmD, University of Missouri-Kansas City School of Pharmacy, Health Sciences Building, Room 2243, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
1. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Brook RA, Forsythe A, Smeeding JE, et al. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813-2821.
3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
4. Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31: 1582-1587.
5. Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3:443-449.
6. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22: 165-172.
7. Dalbeth N, Lindsay K. The patient’s experience of gout: new insights to optimize management. Curr Rheumatol Rep. 2012;14:173-178.
8. Edwards NL. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr Rheumatol Rep. 2011;13:154-159.
9. Singh JA, Hodges JS, Asch SM. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis. 2009;68: 1265-1270.
10. Drugs.com. FDA approves Uloric (febuxostat) for the chronic management of hyperuricemia in patients with gout [press release]. February 13, 2009. Drugs.com Web site. Available at: http://www.drugs.com/newdrugs/fda-approves-uloric-febuxostat-chronic-management-hyperuricemia-patients-gout-1266.html. Accessed October 29, 2014.
11. US Food and Drug Administration. FDA approves new drug for gout [press release]. September 14, 2010. US Food and Drug Administration Web site. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225810.htm. Accessed October 29, 2014.
12. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
13. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
14. Fravel MA, Ernst ME, Clark EC. Gout and hyperuricemia. In: DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw Hill; 2014: 1505-1523.
15. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-452.
16. Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189-192. 17. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
18. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
19. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
20. McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64:121-129.
21. Caspi D, Lubart E, Graff E, et al. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43:103-108.
22. Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060-1068.
23. Wortmann RL, Macdonald PA, Hunt B, et al. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32:2386-2397.
24. US Food and Drug Administration. Uloric (febuxostat tablets). US Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm243770.htm. Accessed October 29, 2014.
25. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
26. Becker MA, Schumacher HR, Benjamin KL, et al; Gout National Study Group. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041-1048.
27. Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad Dermatol. 1979;1:365-374.
28. Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46.
29. Reach G. Treatment adherence in patients with gout. Joint Bone Spine. 2011;78:456-459.
30. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437-443.
31. Lexicomp. News from the world of pharmacology. Lexicomp Web site. Available at: https://www.lexi.com/individuals/pharmacists/newsletters.jsp?id=october_10. Accessed January 13, 2014.
1. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Brook RA, Forsythe A, Smeeding JE, et al. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813-2821.
3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
4. Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31: 1582-1587.
5. Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3:443-449.
6. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22: 165-172.
7. Dalbeth N, Lindsay K. The patient’s experience of gout: new insights to optimize management. Curr Rheumatol Rep. 2012;14:173-178.
8. Edwards NL. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr Rheumatol Rep. 2011;13:154-159.
9. Singh JA, Hodges JS, Asch SM. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis. 2009;68: 1265-1270.
10. Drugs.com. FDA approves Uloric (febuxostat) for the chronic management of hyperuricemia in patients with gout [press release]. February 13, 2009. Drugs.com Web site. Available at: http://www.drugs.com/newdrugs/fda-approves-uloric-febuxostat-chronic-management-hyperuricemia-patients-gout-1266.html. Accessed October 29, 2014.
11. US Food and Drug Administration. FDA approves new drug for gout [press release]. September 14, 2010. US Food and Drug Administration Web site. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225810.htm. Accessed October 29, 2014.
12. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
13. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
14. Fravel MA, Ernst ME, Clark EC. Gout and hyperuricemia. In: DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw Hill; 2014: 1505-1523.
15. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-452.
16. Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189-192. 17. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
18. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
19. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
20. McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64:121-129.
21. Caspi D, Lubart E, Graff E, et al. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43:103-108.
22. Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060-1068.
23. Wortmann RL, Macdonald PA, Hunt B, et al. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32:2386-2397.
24. US Food and Drug Administration. Uloric (febuxostat tablets). US Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm243770.htm. Accessed October 29, 2014.
25. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
26. Becker MA, Schumacher HR, Benjamin KL, et al; Gout National Study Group. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041-1048.
27. Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad Dermatol. 1979;1:365-374.
28. Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46.
29. Reach G. Treatment adherence in patients with gout. Joint Bone Spine. 2011;78:456-459.
30. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437-443.
31. Lexicomp. News from the world of pharmacology. Lexicomp Web site. Available at: https://www.lexi.com/individuals/pharmacists/newsletters.jsp?id=october_10. Accessed January 13, 2014.