User login
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
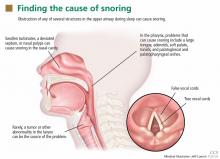
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
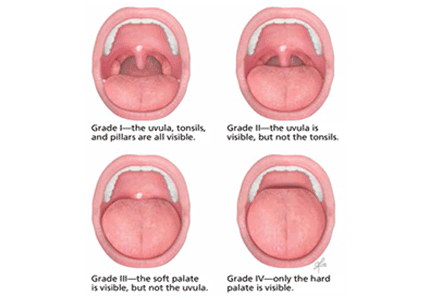
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
Snoring can range in significance from disturbing a bed partner to being a symptom of obstructive sleep apnea, a risk factor for cardiac disease and stroke. Snoring that is unrelated to obstructive sleep apnea may respond to a combination of nonsurgical treatments. However, if the problem persists despite conservative therapy, then surgical options may be considered.
This article explores why people snore, provides guidance for evaluating it and ruling out obstructive sleep apnea, and describes the available surgical treatments. Snoring associated with obstructive sleep apnea requires a different surgical treatment strategy that is beyond the scope of this article.
WHY PEOPLE SNORE
Humans go through four stages of sleep in each sleep cycle (and four or five cycles per night), and each stage has unique physiologic characteristics. As we progress deeper into sleep with each successive stage, the skeletal muscles of the body relax and eventually become atonic, except for the respiratory and ocular muscles. Soft tissues of the upper aerodigestive tract also lose their muscular tone.
Snoring is an undesirable vibratory sound that originates from the soft tissues of the upper respiratory tract during sleep, as airflow causes the relaxed tissues to vibrate.
The upper airway can be obstructed by the nasal septum, inferior nasal turbinates, adenoids, tonsils, uvula, soft palate, and base of the tongue—and often by more than one (Figure 1).3 In rare cases, obstruction can occur at the level of the larynx, such as from a tumor, laryngomalacia, or a laryngeal defect.
A SPECTRUM OF SLEEP-DISORDERED BREATHING
The American Academy of Sleep Medicine’s International Classification of Sleep Disorders1 defines a number of sleep disorders. In clinical practice, the first-line diagnostic test for sleep disorders is polysomnography.
Snoring is only one sign of sleep-disordered breathing; others are excessive daytime somnolence, restless sleep, and witnessed apnea.
Considerable evidence links obstructive sleep apnea with serious medical problems including hypertension, coronary artery disease, heart failure, cardiac arrhythmia, and stroke.2 Others include mood disorders, decreased libido, and cognitive impairment, with changes in attention, concentration, executive function, and fine-motor coordination.4 Therefore, ruling out obstructive sleep apnea is essential before pursuing interventions for primary snoring, although both disorders may warrant surgery.
PATIENT HISTORY
Most patients who present to the office because of snoring have snored for many years. Many seek medical attention at the request of a long-suffering bed partner.
Associated symptoms in primary snoring may include mouth breathing, chronic nasal congestion, and morning dry throat. Witnessed apnea, frequent awakenings during sleep, restless sleep, daytime somnolence, frequents naps, and memory impairment may be signs of more significant sleep-disordered breathing, such as obstructive sleep apnea.
The Epworth sleepiness scale may help quantify the severity of daytime somnolence.5 It is measured in a short questionnaire in which the patient indicates, on a scale of 0 to 3, his or her likelihood of dozing in a variety of situations.
The STOP-BANG questionnaire consists of eight yes-no questions:
- Snore: Have you been told that you snore?
- Tired: Are you often tired during the day?
- Obstruction: Do you know if you stop breathing, or has anyone witnessed you stop breathing while you are asleep?
- Pressure: Do you have high blood pressure or are you on medication to control high blood pressure?
- Body mass index: Is your body mass index higher than 35 kg/m2?
- Age: Are you age 50 or older?
- Neck: Do you have a neck circumference greater than 17 inches (men) or greater than 16 inches (women)?
- Gender: Are you male?
A score of three or higher has shown a sensitivity of 93% for detecting moderate obstructive sleep apnea and 100% for severe obstructive sleep apnea.6
SEARCHING FOR ANATOMIC CAUSES OF SNORING
A thorough physical examination should be done, focusing on potential anatomic causes of snoring. Nasal septal deviation or inferior turbinate hypertrophy with mucosal congestion may contribute to chronic mouth breathing secondary to nasal obstruction. Patients with a body mass index over 35 kg/m2 and neck circumference over 17 inches (16 inches in women) are at higher risk of obstructive sleep apnea.
Indirect mirror examination or flexible transnasal endoscopy may reveal obstructing or persistent adenoid lymphoid tissue, particularly in young adults. Transnasal endoscopy may also reveal dynamic collapse of the palate and lateral oropharyngeal wall or fullness of the tongue base with subsequent narrowing of the oropharynx.
Examination of the oral cavity may reveal a disproportionately large tongue, a narrow opening into the oropharynx, or tonsillar hypertrophy. The Friedman classification (Figure 2), also called the modified Mallampati scale, can be used to describe the findings on physical examination of the palate and tongue in a systematic way. There are four grades of increasing severity, and the higher the grade, the less likely that surgery will succeed in patients with obstructive sleep apnea.7 The mouth is examined with the tongue in a relaxed position; in contrast, the original Mallampati classification, which is often used by anesthesiologists in assessing the oral airway, is assessed with the tongue protruding.
During flexible endoscopy, asking the patient to attempt to recreate the snoring can sometimes reveal the causative anatomic structure, which is usually the soft palate.
SLEEP STUDIES
A full diagnostic workup should include a sleep study if obstructive sleep apnea cannot be ruled out by the history and examination. Sleep studies include either polysomnography in a sleep laboratory or a home sleep test. They allow the clinician to further evaluate the severity of sleep-disordered breathing and to distinguish primary snoring from obstructive sleep apnea. This is particularly important if elective surgical intervention is planned. Sleep studies can also be used to evaluate for other sleep disorders.
Apnea is considered obstructive when polysomnography reveals episodes of no oral or nasal airflow with continued inspiratory effort, evidenced by abdominal or thoracic muscle activity. Hypopnea is defined as a 30% or greater reduction in airflow lasting at least 10 seconds, with an associated 4% or greater oxygen desaturation.1 The combined number of apnea and hypopnea events per hour, or apnea-hypopnea index, is used clinically to quantify the severity of sleep-disordered breathing.
Primary snoring is diagnosed if the apnea-hypopnea index is 5 or less. Obstructive sleep apnea is considered mild when the apnea-hypopnea index is greater than 5 but less than 15, moderate from 15 to 30, and severe if over 30.
LIMITED ROLE FOR IMAGING
Cephalometric radiography (plain radiography of the airways) has limited value in the workup of primary snoring and is discouraged. Imaging is most useful in assessing craniofacial skeletal abnormalities. Lateral airway images can help in diagnosing adenoid hypertrophy in children. However, flexible nasopharyngoscopy can obtain this information by direct visualization with no radiation exposure.
Computed tomography and magnetic resonance imaging are seldom used in the workup of snoring because they do little to guide therapeutic intervention, are expensive, and, in the case of computed tomography, expose the patient to unnecessary radiation. Imaging does a have a role when planning surgical intervention of obstruction that involves the maxillofacial skeleton.
NONSURGICAL MANAGEMENT
The primary goal of therapy for snoring is to eliminate or reduce noise levels.
Although no study to date has analyzed the efficacy of nonsurgical management, several treatments are aimed at the root causes of snoring in an attempt to decrease it.
Intranasal topical steroids reduce inflammation of the nasal mucosa that occurs with allergic and nonallergic rhinitis, thereby opening up the nasal airway. They may reduce snoring in a small number of cases. These drugs must often be used in the long term to maintain their efficacy.
Devices. Other than continuous positive airway pressure (CPAP), the only currently available nonsurgical device approved by the US Food and Drug Administration for the treatment of snoring and obstructive sleep apnea is an oral dental appliance, which is customized to the patient’s dentition to relieve upper-airway obstruction by soft tissues of the oral cavity. The lower jaw is forced anteriorly, pulling the tongue and attached soft tissues forward. Custom-fitted oral appliances are an effective option for mild to moderate sleep apnea and associated snoring, and are more effective than thermoplastic “boil-and-bite” devices.8 These can easily be used in patients who have primary snoring.
Over-the-counter remedies such as nasal strips and head-positioning pillows have not been shown to be efficacious for snoring.9
Weight loss. Patients should be encouraged to join a weight-management program if overweight.
Sleep on the side, not on the back. Changing the sleep position may be useful in patients who have positional symptoms. Snoring is often worse in the supine position because gravity acting on the palate and tongue causes narrowing of the airway. “Positional therapy” employs devices to force patients to sleep in a lateral decubitus position to counter the effects of gravity.
Alcohol cessation. Alcohol has a relaxing effect on the muscles of the upper-respiratory tract, and abstaining from alcohol may therefore reduce snoring.
INDICATIONS FOR SURGERY
Surgery can decrease the noise level of snoring and thus bring relief for the patient’s bed partner.
Assessment of the upper airway may suggest the appropriate treatment, depending on whether the patient has nasal obstruction, adenoid hypertrophy, or palatal movement. A sleep study, if not previously done, should be done before surgery to rule out obstructive sleep apnea.
Many patients opt for surgery after noninvasive forms of treatment have proven ineffective or difficult to tolerate. When medical therapy for snoring has been unsuccessful, a discussion of the benefits, risks, and alternatives to surgery must take place between the patient and the surgeon.
SURGICAL PROCEDURES
Septoplasty
Septoplasty—straightening the nasal septum to improve the nasal airway—is an outpatient procedure. Although a deviated septum alone is not often the sole cause of snoring, most otolaryngologists agree that the septum should be addressed before or concomitantly with any palatal surgery for sleep-disordered breathing.
Nasal congestion often comes from a deviated bony or cartilaginous septum, enlarged turbinates, or bone spurs. Septal deviation may be developmental or the result of trauma to the nose.
Complications of septoplasty are rare but include septal perforation, scar-band formation, septal hematoma, epistaxis, and infection.
Radiofrequency ablation of the inferior turbinates
Hypertrophy of the inferior turbinate is the most common cause of nasal obstruction, followed by structural deformity of the nasal airway by septal deviation.3 Many patients report fixed or fluctuating nasal congestion and chronic mouth-breathing. The causes of turbinate congestion or enlargement include allergic rhinitis, upper-respiratory infection, and chronic rhinitis. In most cases, turbinate hypertrophy occurs at the level of the submucosa.
Radiofrequency ablation uses radiofrequency energy to generate heat at approximately 85°C (185°F) to create finely controlled coagulative lesions. The lesions are naturally resorbed in 3 to 8 weeks, inducing fibrosis, reducing excess tissue volume, and thus opening the airway. The procedure can be repeated several times to achieve optimal results. Radiofrequency ablation can also be used to reduce anatomic obstruction in other parts of the airway, such as the soft palate and the base of tongue.
Submucosal radiofrequency ablation of the inferior turbinate is a simple office-based procedure. It is often combined with septoplasty to optimize the nasal airway.
Mild to moderate edema with subsequent nasal obstruction and thick mucus formation can be expected the first week after the procedure. The risk of postoperative bleeding and infection is low. When performed with septoplasty, there is a low risk that scar tissue, or synechiae, may form between the turbinate and the septum.
Radiofrequency ablation of the palate
The soft palate is the most common anatomic source of snoring, and radiofrequency ablation can be applied to it as well. As with radiofrequency ablation in other areas, coagulative necrosis leads to fibrosis, and the soft tissue eventually contracts in volume with increased stiffness, thereby resulting in less tissue elasticity and vibration.
Carroll et al10 reported that nasal surgery combined with radiofrequency ablation of either the palate or the base of the tongue completely resolved snoring (according to the patient’s bed partner) in 42% of cases and improved it in 52%, with few complications. Also, patients who received more than one radiofrequency ablation application were more than twice as likely to have resolution of their snoring.
A systematic review of palatal radiofrequency ablation for snoring found that it is safe with minimal complication rates and reduces snoring in short-term follow-up.11 The authors reviewed 30 studies: two randomized controlled trials, four clinical controlled trials, and 24 prospective uncontrolled studies. The only placebo-controlled randomized controlled trial found soft-palate radiofrequency ablation to be superior to placebo. In these studies, follow-up varied from 6 weeks to 26 months. However, the relapse rate was as high as 50% at a mean follow-up time of 13.2 months.
Thus, most of the information in this review has come from observational studies with short follow-up time. In another study, however, the authors presented a 5-year follow-up of palatal radiofrequency ablation that showed persistent and satisfying reduction of snoring.12
Injection snoreplasty
Alternative procedures have been used to reduce palatal flutter that leads to snoring.
Injection snoreplasty was first described by Brietzke and Mair al in 2001.13 Sodium tetradecyl sulfate, a sclerotherapy agent, is injected directly into the submucosal layer of the soft palate to induce scarring and reduce or eliminate snoring caused by the soft palate.
In a cohort study of 25 patients, the subjective success rate was 75% (13 patients) as far out as 19 months.14 In a separate cohort of 17 patients, home polysomnography with audio recordings was done before and after treatment in patients who underwent injection snoreplasty. Twelve (17%) of these patients had a significant reduction in the proportion of palatal snoring, loudness, and flutter frequency. Long-term success and snoring relapse rates of injection snoreplasty were reported to be similar to those of other current treatments.14
Pillar implants
The Pillar implant (Medtronic) was approved by the US Food and Drug Administration in 2002 for snoring and in 2004 for mild to moderate obstructive sleep apnea.
The implant, made of a woven polyester material, is designed to reduce vibration of the soft palate by increasing its stiffness. The implant induces a chronic inflammatory response that is thought to result in the formation of a fibrous capsule, which may also play a role in palatal stiffening. Three thin implants are inserted into the paramedian soft palate in a parallel orientation. This is an outpatient procedure done in the office.
The short-term benefits of the Pillar implant procedure have been well documented.15,16 A meta-analysis of seven case-controlled studies that included 174 patients found the Pillar implant significantly decreased the loudness of snoring by 59%.15 The major disadvantage of Pillar implants was their high extrusion rate, which was reported to be 9.3%.15 While statistically significant improvement has been shown at up to 1 year, a recent longitudinal study suggests a clinical deterioration in snoring scale scores by 4 years after the procedure.16
Laser-assisted uvulopalatoplasty
Laser-assisted uvulopalatoplasty is a staged office-based procedure that involves removal of excess uvular mucosa and the creation of transpalatal vertical troughs to widen the retropalatal airway for the treatment of snoring and mild obstructive sleep apnea. The treatment typically requires about three sessions. It aims to mimic the palatal appearance of uvulopalatopharyngoplasty used to treat obstructive sleep apnea and has been proposed to have similar surgical outcomes in properly selected patients.
Krespi and Kaeker,17 in 1994, were among the first to describe the technique in the United States.
Kyrmizakis et al,18 in a retrospective study of 59 patients with habitual snoring who underwent laser-assisted uvulopalatoplasty, showed that a significant number of patients benefited from the procedure. During a follow-up ranging from 6 months to 5 years (mean 40 months), 91.5% of the patients with habitual snoring reported significant short-term improvement based on a posttreatment questionnaire, and 79.7% reported long-term subjective improvement.
Unfortunately, most of the studies have been small, and thus there is some controversy about the efficacy of laser-assisted uvulopalatoplasty, particularly in patients with obstructive sleep apnea. The most significant complication during healing is pain, which may deter patients from completing the full course of treatment.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders – Second Edition (ICSD-2). American Academy of Sleep Medicine 2005, 0965722023 978-0965722025.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914.
- Clemente CD. Anatomy: A Regional Atlas of the Human Body. Philadelphia; Lippincott Williams & Wilkins; 2010:752.
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011; 190:53–68.
- Damiani MF, Quaranta VN, Falcone VA, et al. The Epworth Sleepiness Scale: conventional self vs physician administration. Chest 2013; 143:1569–1575.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002; 127:13–21.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197–202.
- Michaelson P, Mair EA. Popular snore aids: do they work? Otolaryngol Head Neck Surg 2004; 130:649–658.
- Carroll W, Wilhoit CS, Intaphan J, Nguyen SA, Gillespie MB. Snoring management with nasal surgery and upper airway radiofrequency ablation. Otolaryngol Head Neck Surg 2012; 146:1023–1027.
- Bäck LJ, Hytönen ML, Roine RP, Malmivaara AOV. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009, 119:1241–1250.
- DeVito A, Frassinet S, Panatta ML, Montevecchi F, Canzi P, Vicini C. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otolaryngol 2012; 269:321–330.
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001; 124:503–510.
- Brietzke SE, Mair EA. Injection snoreplasty: extended follow-up and new objective data. Otolaryngol Head Neck Surg 2003; 128:605–615.
- Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013; 123:269–276.
- Rotenberg BW, Luu K. Four-year outcomes of palatal implants for primary snoring treatment: a prospective longitudinal study. Laryngoscope 2012; 122:696–699.
- Krespi YP, Kacker A. Laser-assisted uvulopalatoplasty revisited. Otolaryngol Clin North Am 2003; 36:495–500.
- Kyrmizakis DE, Chimona TS, Papadakis CE, et al. Laser-assisted uvulopalatoplasty for the treatment of snoring and mild obstructive sleep apnea syndrome. J Otolaryngol 2003; 32:174–179.
KEY POINTS
- The treatment of snoring begins with a thorough history and physical examination.
- Polysomnography is almost always necessary to rule out other sleep disorders, such as obstructive sleep apnea. This is particularly important if an elective surgical intervention is planned.
- Surgical procedures for snoring include septoplasty with or without radiofrequency ablation of the upper airway, injection snoreplasty, Pillar implants, and laser-assisted uvulopalatoplasty.
- Although studies indicate that these procedures are effective, no well-controlled study has compared one procedure against another. The choice of procedure is often determined by the expertise of the surgeon, and the outcome is highly dependent on the skill of the surgeon.