User login
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
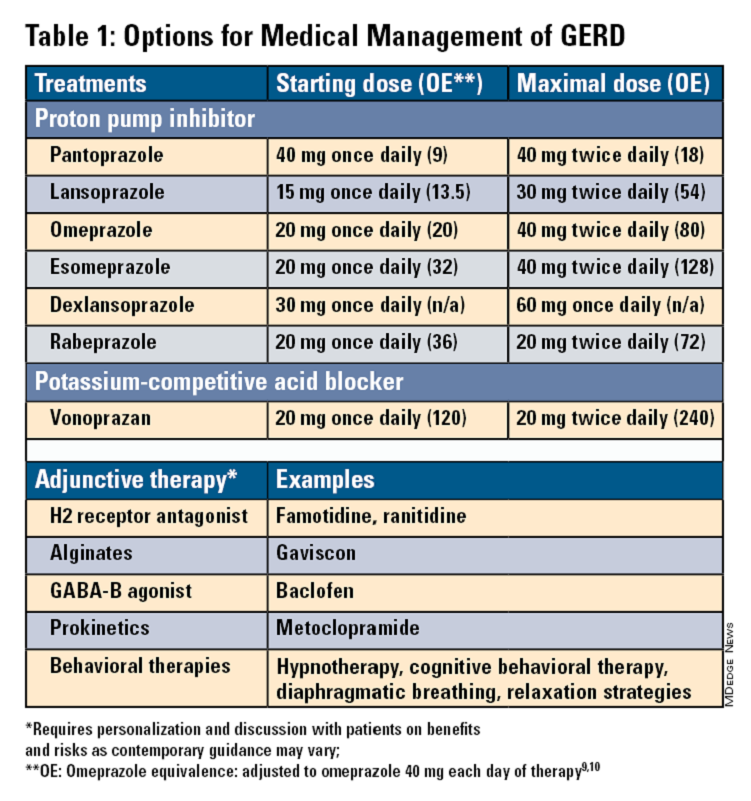
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8
Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
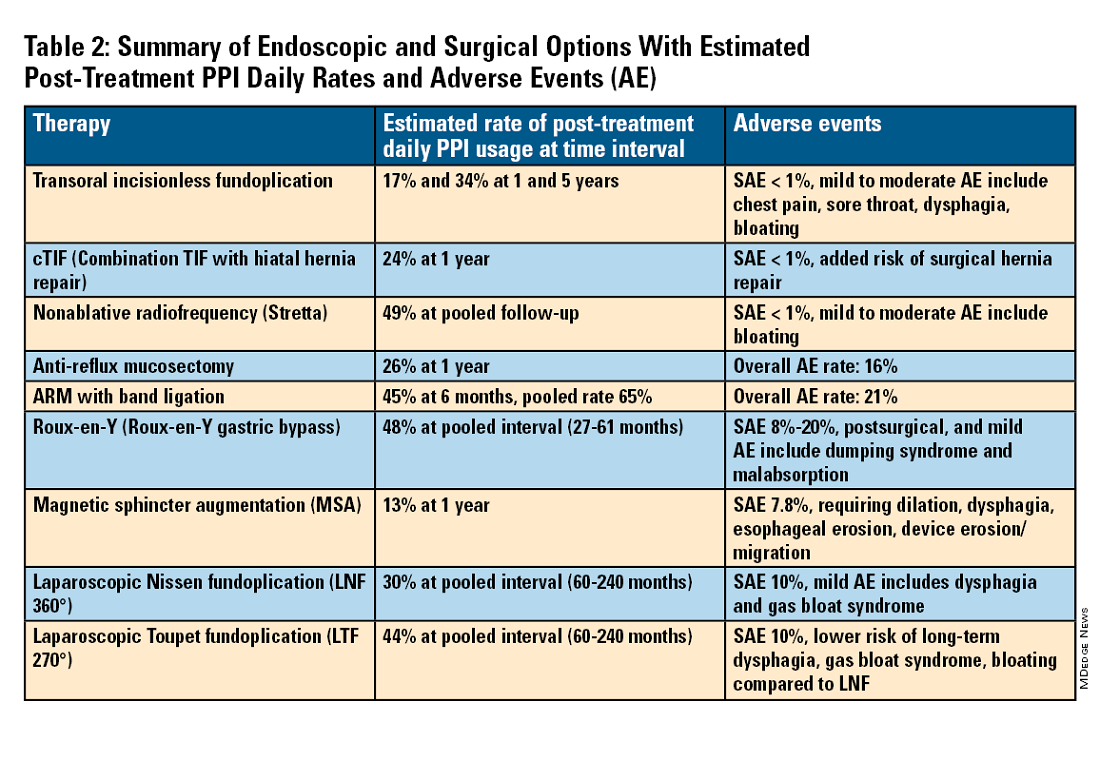
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.
The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.



