User login
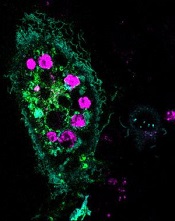
engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()

engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()

engulfed dying cells (purple)
Credit: Toru Komatsu
A two-pronged approach can prompt phagocytosis in inert cells, according to a paper published in Science Signaling.
Researchers manipulated HeLa cells, which typically cannot perform phagocytosis, by activating one protein inside the cells and expressing another protein on the cells’ surface. This forced the cells to engulf apoptotic Jurkat T cells.
So the researchers believe this technique could be used as a targeted therapy, with engineered cells consuming unwanted cells.
“Our goal is to build artificial cells programmed to eat up dangerous junk in the body, which could be anything from bacteria to the amyloid-beta plaques that cause Alzheimer’s to the body’s own rogue cancer cells,” said study author Takanari Inoue, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“By figuring out how to get normally inert cells to recognize and engulf dying cells, we’ve taken an important step in that direction.”
Dr Inoue and his colleagues set out to “strip down” phagocytosis, determining the minimum tools one cell needs to eat another. Their first task was to induce the HeLa cells to attach to nearby dying cells—apoptotic Jurkat T cells—by getting the right receptors to the HeLa cells’ surface.
The researchers knew that part of a receptor protein called MFG-E8 would recognize and stick to a distress signal on the surface of dying cells, and coaxing the HeLa cells to make the protein fragment was straightforward.
To get the fragment, termed C2, onto the outside of the cells, the team found a way to stick it to another protein that was bound for the cell’s surface, thus taking advantage of the cell’s own transportation system.
As a result, up to 6 apoptotic Jurkat T cells stuck to each HeLa cell. The bad news was that the HeLa cells weren’t actually eating the T cells.
Fortunately, the researchers already had an idea about what to try next. Previous research had shown that activating the Rac gene could cause a cell to engulf beads stuck to its surface.
Sure enough, the team found that HeLa cells with both surface C2 and activated Rac swallowed the apoptotic cells readily.
“We’ve shown it’s possible to endow ordinary cells with the power to do something unique: take on the role of a specialized macrophage,” Dr Inoue said.
He cautioned, however, that the researchers don’t believe the engulfed cells are being broken down. Getting the HeLa cells to finish the process of phagocytosis will be one of the group’s next steps. ![]()