User login
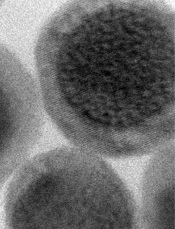
Credit: Jonathan Lovell
A new type of nanoparticle can be used with 6 different imaging techniques, according to research published in Advanced Materials.
Investigators found they could detect these nanoparticles via CT and PET scans, as well as photoacoustic, fluorescence, upconversion, and Cerenkov luminescence imaging.
A machine capable of performing all 6 imaging techniques at once has not yet been invented, to the researchers’ knowledge.
But they hope the creation of their nanoparticles and related work will spur the development of such technology.
That way, patients could receive a single injection of the nanoparticles and have several types of imaging done, which would provide a clearer picture of organs and tissues than a single imaging method alone.
For instance, when the investigators used their nanoparticles to examine the lymph nodes of mice, they found that CT and PET scans provided the
deepest tissue penetration, while the photoacoustic imaging showed blood vessel details the first 2 techniques missed.
“This nanoparticle may open the door for new ‘hypermodal’ imaging systems that allow a lot of new information to be obtained using just one contrast agent,” said study author Jonathan Lovell, PhD, of the University of Buffalo in New York.
“Once such systems are developed, a patient could theoretically go in for one scan with one machine instead of multiple scans with multiple machines.”
Dr Lovell and his colleagues designed their nanoparticles to have 2 components: a core that glows blue when struck by near-infrared light and an outer fabric of porphyrin-phospholipids (PoP) that wraps around the core.
Each part has unique characteristics that make it ideal for certain types of imaging.
The core, initially designed for upconversion imaging, is made from sodium, ytterbium, fluorine, yttrium, and thulium. The ytterbium is dense in electrons—a property that facilitates detection by CT scans.
The PoP wrapper has biophotonic qualities that make it a great match for fluorescence and photoacoustic imagining. The PoP layer is also adept at attracting copper, which is used in PET and Cerenkov luminescence imaging.
“Combining these 2 biocompatible components into a single nanoparticle could give tomorrow’s doctors a powerful new tool for medical imaging,” said Paras Prasad, PhD, also of the University of Buffalo.
“More studies would have to be done to determine whether the nanoparticle is safe to use for such purposes, but it does not contain toxic metals, such as cadmium, that are known to pose potential risks and are found in some other nanoparticles.”
“Another advantage of this core/shell imaging contrast agent is that it could enable biomedical imaging at multiple scales, from single-molecule to cell imaging, as well as from vascular and organ imaging to whole-body bioimaging,” added Guanying Chen, PhD, of the University of Buffalo and Harbin Institute of Technology in China.
Dr Lovell said the next step for this research is to explore additional uses for the technology.
For example, it might be possible to attach a targeting molecule to the PoP surface that would enable cancer cells to take up the particles, something that photoacoustic and fluorescence imaging can detect due to the properties of the smart PoP coating. This would enable doctors to better see where tumors begin and end. ![]()

Credit: Jonathan Lovell
A new type of nanoparticle can be used with 6 different imaging techniques, according to research published in Advanced Materials.
Investigators found they could detect these nanoparticles via CT and PET scans, as well as photoacoustic, fluorescence, upconversion, and Cerenkov luminescence imaging.
A machine capable of performing all 6 imaging techniques at once has not yet been invented, to the researchers’ knowledge.
But they hope the creation of their nanoparticles and related work will spur the development of such technology.
That way, patients could receive a single injection of the nanoparticles and have several types of imaging done, which would provide a clearer picture of organs and tissues than a single imaging method alone.
For instance, when the investigators used their nanoparticles to examine the lymph nodes of mice, they found that CT and PET scans provided the
deepest tissue penetration, while the photoacoustic imaging showed blood vessel details the first 2 techniques missed.
“This nanoparticle may open the door for new ‘hypermodal’ imaging systems that allow a lot of new information to be obtained using just one contrast agent,” said study author Jonathan Lovell, PhD, of the University of Buffalo in New York.
“Once such systems are developed, a patient could theoretically go in for one scan with one machine instead of multiple scans with multiple machines.”
Dr Lovell and his colleagues designed their nanoparticles to have 2 components: a core that glows blue when struck by near-infrared light and an outer fabric of porphyrin-phospholipids (PoP) that wraps around the core.
Each part has unique characteristics that make it ideal for certain types of imaging.
The core, initially designed for upconversion imaging, is made from sodium, ytterbium, fluorine, yttrium, and thulium. The ytterbium is dense in electrons—a property that facilitates detection by CT scans.
The PoP wrapper has biophotonic qualities that make it a great match for fluorescence and photoacoustic imagining. The PoP layer is also adept at attracting copper, which is used in PET and Cerenkov luminescence imaging.
“Combining these 2 biocompatible components into a single nanoparticle could give tomorrow’s doctors a powerful new tool for medical imaging,” said Paras Prasad, PhD, also of the University of Buffalo.
“More studies would have to be done to determine whether the nanoparticle is safe to use for such purposes, but it does not contain toxic metals, such as cadmium, that are known to pose potential risks and are found in some other nanoparticles.”
“Another advantage of this core/shell imaging contrast agent is that it could enable biomedical imaging at multiple scales, from single-molecule to cell imaging, as well as from vascular and organ imaging to whole-body bioimaging,” added Guanying Chen, PhD, of the University of Buffalo and Harbin Institute of Technology in China.
Dr Lovell said the next step for this research is to explore additional uses for the technology.
For example, it might be possible to attach a targeting molecule to the PoP surface that would enable cancer cells to take up the particles, something that photoacoustic and fluorescence imaging can detect due to the properties of the smart PoP coating. This would enable doctors to better see where tumors begin and end. ![]()

Credit: Jonathan Lovell
A new type of nanoparticle can be used with 6 different imaging techniques, according to research published in Advanced Materials.
Investigators found they could detect these nanoparticles via CT and PET scans, as well as photoacoustic, fluorescence, upconversion, and Cerenkov luminescence imaging.
A machine capable of performing all 6 imaging techniques at once has not yet been invented, to the researchers’ knowledge.
But they hope the creation of their nanoparticles and related work will spur the development of such technology.
That way, patients could receive a single injection of the nanoparticles and have several types of imaging done, which would provide a clearer picture of organs and tissues than a single imaging method alone.
For instance, when the investigators used their nanoparticles to examine the lymph nodes of mice, they found that CT and PET scans provided the
deepest tissue penetration, while the photoacoustic imaging showed blood vessel details the first 2 techniques missed.
“This nanoparticle may open the door for new ‘hypermodal’ imaging systems that allow a lot of new information to be obtained using just one contrast agent,” said study author Jonathan Lovell, PhD, of the University of Buffalo in New York.
“Once such systems are developed, a patient could theoretically go in for one scan with one machine instead of multiple scans with multiple machines.”
Dr Lovell and his colleagues designed their nanoparticles to have 2 components: a core that glows blue when struck by near-infrared light and an outer fabric of porphyrin-phospholipids (PoP) that wraps around the core.
Each part has unique characteristics that make it ideal for certain types of imaging.
The core, initially designed for upconversion imaging, is made from sodium, ytterbium, fluorine, yttrium, and thulium. The ytterbium is dense in electrons—a property that facilitates detection by CT scans.
The PoP wrapper has biophotonic qualities that make it a great match for fluorescence and photoacoustic imagining. The PoP layer is also adept at attracting copper, which is used in PET and Cerenkov luminescence imaging.
“Combining these 2 biocompatible components into a single nanoparticle could give tomorrow’s doctors a powerful new tool for medical imaging,” said Paras Prasad, PhD, also of the University of Buffalo.
“More studies would have to be done to determine whether the nanoparticle is safe to use for such purposes, but it does not contain toxic metals, such as cadmium, that are known to pose potential risks and are found in some other nanoparticles.”
“Another advantage of this core/shell imaging contrast agent is that it could enable biomedical imaging at multiple scales, from single-molecule to cell imaging, as well as from vascular and organ imaging to whole-body bioimaging,” added Guanying Chen, PhD, of the University of Buffalo and Harbin Institute of Technology in China.
Dr Lovell said the next step for this research is to explore additional uses for the technology.
For example, it might be possible to attach a targeting molecule to the PoP surface that would enable cancer cells to take up the particles, something that photoacoustic and fluorescence imaging can detect due to the properties of the smart PoP coating. This would enable doctors to better see where tumors begin and end. ![]()