User login
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
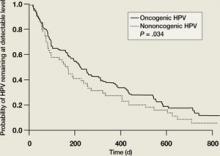
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
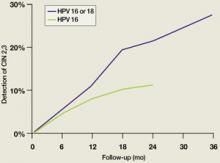
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.