User login
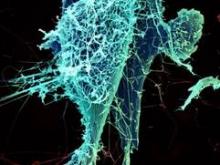
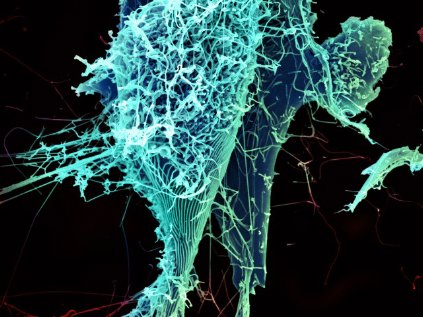
The National Institute of Allergy and Infectious Diseases will begin human testing of a new vaccine developed to prevent contracting the deadly Ebola virus.
The vaccine is the first of several phase I treatments to go into clinical trials on humans. The hope is that the vaccine ultimately will be used to stem the tide of Ebola infections in western Africa, where the virus already has claimed more than 1,400 lives since the outbreak began in March.
"There is an urgent need for a protective Ebola vaccine, and it is important to establish that a vaccine is safe and spurs the immune system to react in a way necessary to protect against infection," Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a statement. "The [National Institutes of Health] is playing a key role in accelerating the development and testing of investigational Ebola vaccines."
The testing will take place at the National Institutes of Health Clinical Center in Maryland, where the vaccine – developed jointly by the NIAID and GlaxoSmithKline – will be administered to 20 healthy adults who have not contracted the Ebola virus. Ten adults will receive an intramuscular injection, while the other ten will receive the same injection in a higher dosage. All participants in the trial, which has been dubbed VRC 207, are between the ages of 18 and 50.
Once phase I trials are complete, the next step will be to test the vaccine on a larger number of people in phase II studies. Phase III trials would determine whether the vaccine is effective. The VRC 207 trial is expected to begin in October.
"Today we know the best way to prevent the spread of Ebola infection is through public health measures, including good infection control practices, isolation, contact tracing, quarantine, and provision of personal protective equipment," Dr. Fauci said in the statement. "However, a vaccine will ultimately be an important tool in the prevention effort. The launch of phase I Ebola vaccine studies is the first step in a long process."
Additionally, the NIH has partnered with a British consortium to test the NIAID/GSK vaccine on healthy adults in the United Kingdom. Permission to test the vaccine in the western African nations of Gambia and Mali is still pending approval from authorities in those countries. The U.K. trial is scheduled to begin in September.
The NIAID anticipates that initial safety and immunogenicity data from the phase I trials should be available by late 2014.
The National Institute of Allergy and Infectious Diseases will begin human testing of a new vaccine developed to prevent contracting the deadly Ebola virus.
The vaccine is the first of several phase I treatments to go into clinical trials on humans. The hope is that the vaccine ultimately will be used to stem the tide of Ebola infections in western Africa, where the virus already has claimed more than 1,400 lives since the outbreak began in March.
"There is an urgent need for a protective Ebola vaccine, and it is important to establish that a vaccine is safe and spurs the immune system to react in a way necessary to protect against infection," Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a statement. "The [National Institutes of Health] is playing a key role in accelerating the development and testing of investigational Ebola vaccines."
The testing will take place at the National Institutes of Health Clinical Center in Maryland, where the vaccine – developed jointly by the NIAID and GlaxoSmithKline – will be administered to 20 healthy adults who have not contracted the Ebola virus. Ten adults will receive an intramuscular injection, while the other ten will receive the same injection in a higher dosage. All participants in the trial, which has been dubbed VRC 207, are between the ages of 18 and 50.
Once phase I trials are complete, the next step will be to test the vaccine on a larger number of people in phase II studies. Phase III trials would determine whether the vaccine is effective. The VRC 207 trial is expected to begin in October.
"Today we know the best way to prevent the spread of Ebola infection is through public health measures, including good infection control practices, isolation, contact tracing, quarantine, and provision of personal protective equipment," Dr. Fauci said in the statement. "However, a vaccine will ultimately be an important tool in the prevention effort. The launch of phase I Ebola vaccine studies is the first step in a long process."
Additionally, the NIH has partnered with a British consortium to test the NIAID/GSK vaccine on healthy adults in the United Kingdom. Permission to test the vaccine in the western African nations of Gambia and Mali is still pending approval from authorities in those countries. The U.K. trial is scheduled to begin in September.
The NIAID anticipates that initial safety and immunogenicity data from the phase I trials should be available by late 2014.
The National Institute of Allergy and Infectious Diseases will begin human testing of a new vaccine developed to prevent contracting the deadly Ebola virus.
The vaccine is the first of several phase I treatments to go into clinical trials on humans. The hope is that the vaccine ultimately will be used to stem the tide of Ebola infections in western Africa, where the virus already has claimed more than 1,400 lives since the outbreak began in March.
"There is an urgent need for a protective Ebola vaccine, and it is important to establish that a vaccine is safe and spurs the immune system to react in a way necessary to protect against infection," Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a statement. "The [National Institutes of Health] is playing a key role in accelerating the development and testing of investigational Ebola vaccines."
The testing will take place at the National Institutes of Health Clinical Center in Maryland, where the vaccine – developed jointly by the NIAID and GlaxoSmithKline – will be administered to 20 healthy adults who have not contracted the Ebola virus. Ten adults will receive an intramuscular injection, while the other ten will receive the same injection in a higher dosage. All participants in the trial, which has been dubbed VRC 207, are between the ages of 18 and 50.
Once phase I trials are complete, the next step will be to test the vaccine on a larger number of people in phase II studies. Phase III trials would determine whether the vaccine is effective. The VRC 207 trial is expected to begin in October.
"Today we know the best way to prevent the spread of Ebola infection is through public health measures, including good infection control practices, isolation, contact tracing, quarantine, and provision of personal protective equipment," Dr. Fauci said in the statement. "However, a vaccine will ultimately be an important tool in the prevention effort. The launch of phase I Ebola vaccine studies is the first step in a long process."
Additionally, the NIH has partnered with a British consortium to test the NIAID/GSK vaccine on healthy adults in the United Kingdom. Permission to test the vaccine in the western African nations of Gambia and Mali is still pending approval from authorities in those countries. The U.K. trial is scheduled to begin in September.
The NIAID anticipates that initial safety and immunogenicity data from the phase I trials should be available by late 2014.