User login
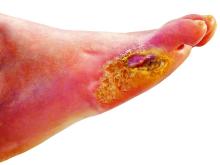
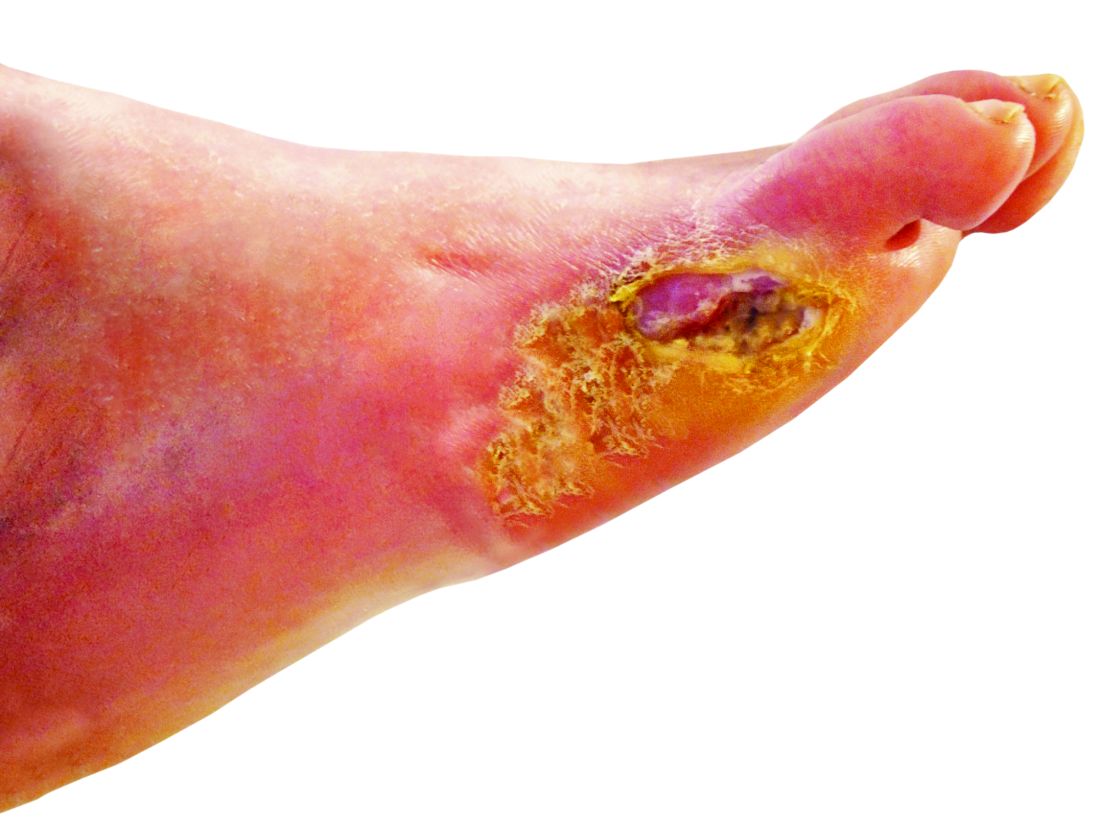
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
FROM WOUND REPAIR AND REGENERATION