User login
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
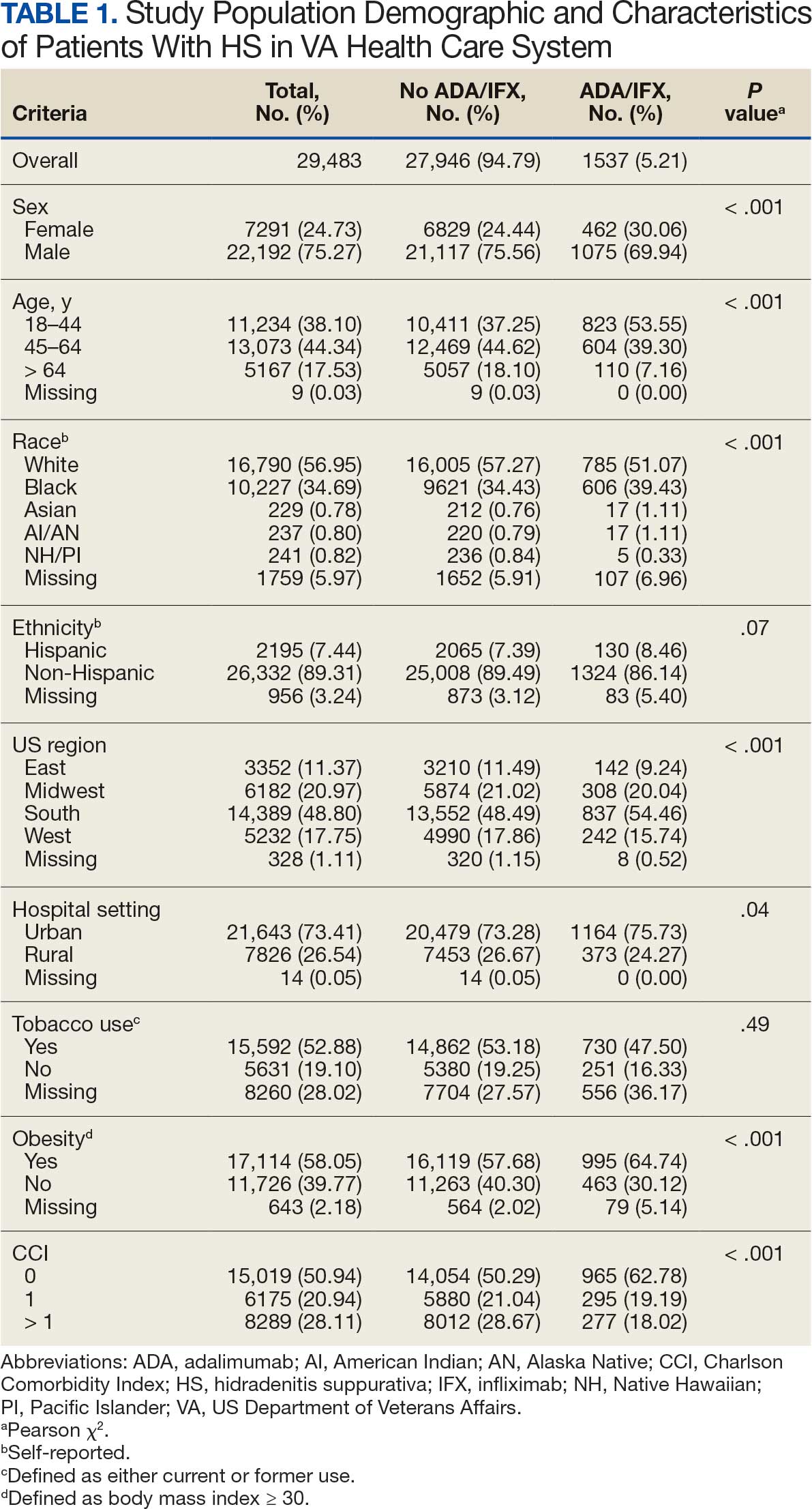
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).
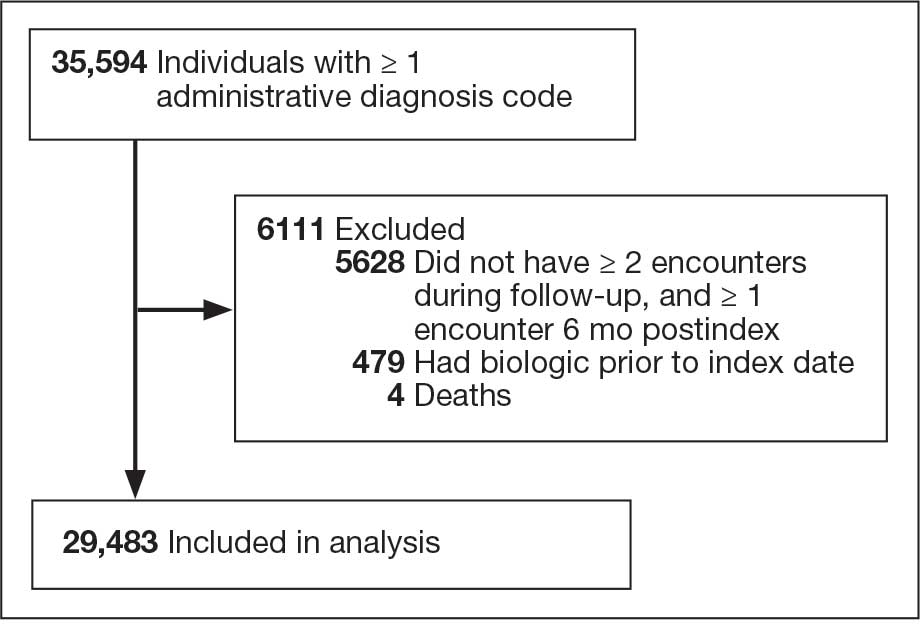
Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7
Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Richard P. Usatine, MD
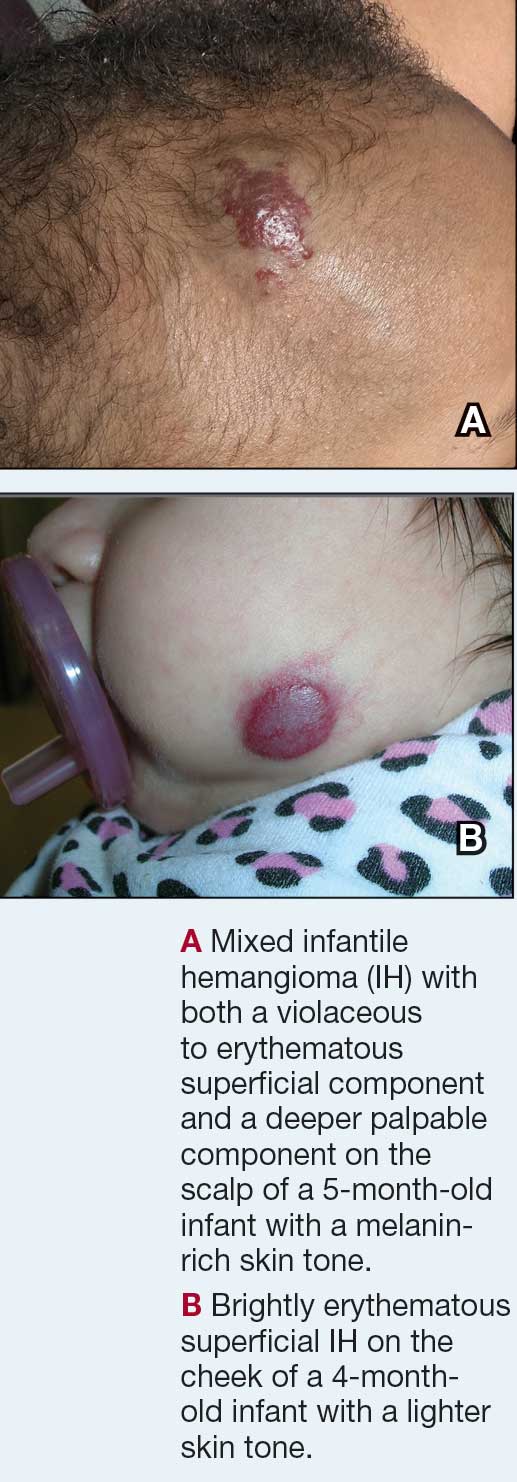
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
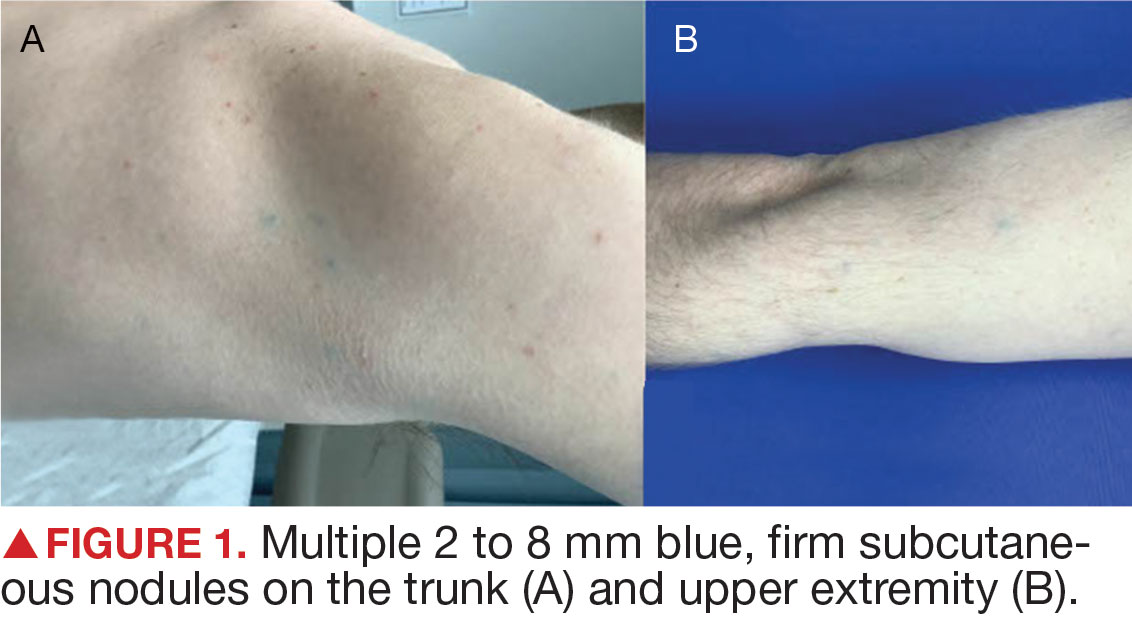
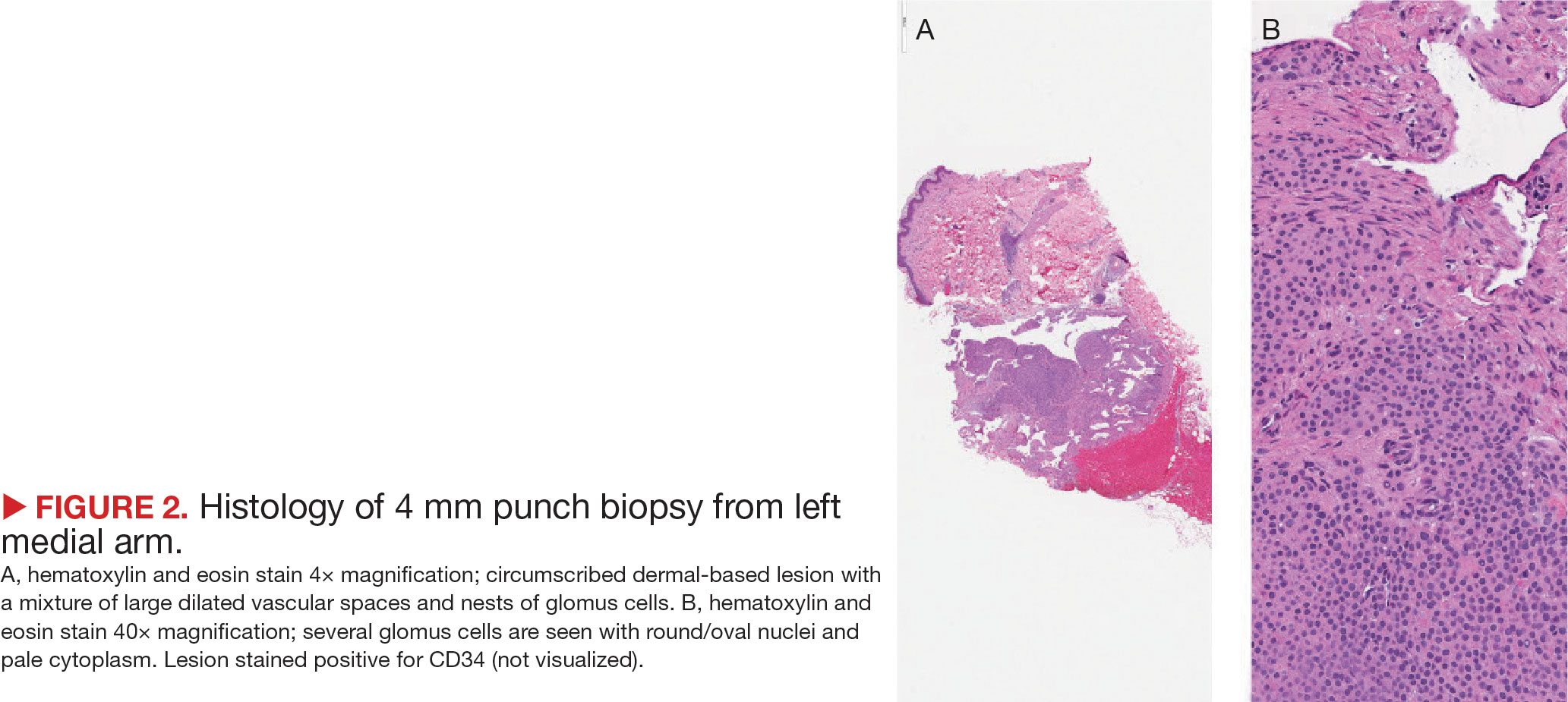
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.

Richard P. Usatine, MD
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.

Richard P. Usatine, MD
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
A 62-year-old woman presented for a fullbody skin examination and was found to have a rash in her axillae and inframammary regions. The rash was intermittently pruritic, and the patient felt that the inframammary rash had started from contact with brassiere underwires. She had no oral lesions but noted intermittent burning and itching of the vaginal folds and intermittent bleeding near her anus. Physical examination revealed confluent, shiny, white, atrophic, thin papules with surrounding pink and purple patches on bilateral axillae, bilateral inframammary folds, bilateral inner thighs, and on the clitoral hood and labia minora. There was also an hourglass-shaped erythematous patch involving the vagina and anus. A small fissure was noted perianally, and small hemorrhage was noted on the clitoral head, with fusion of the clitoral head and superior labia minora (Figures 1 and 2).
lesion from punch biopsy of the patient’s left axilla.
sclerosus plaque showing bright white grouped dots
on a pink background with follicular plugging and linear
branching vessels.
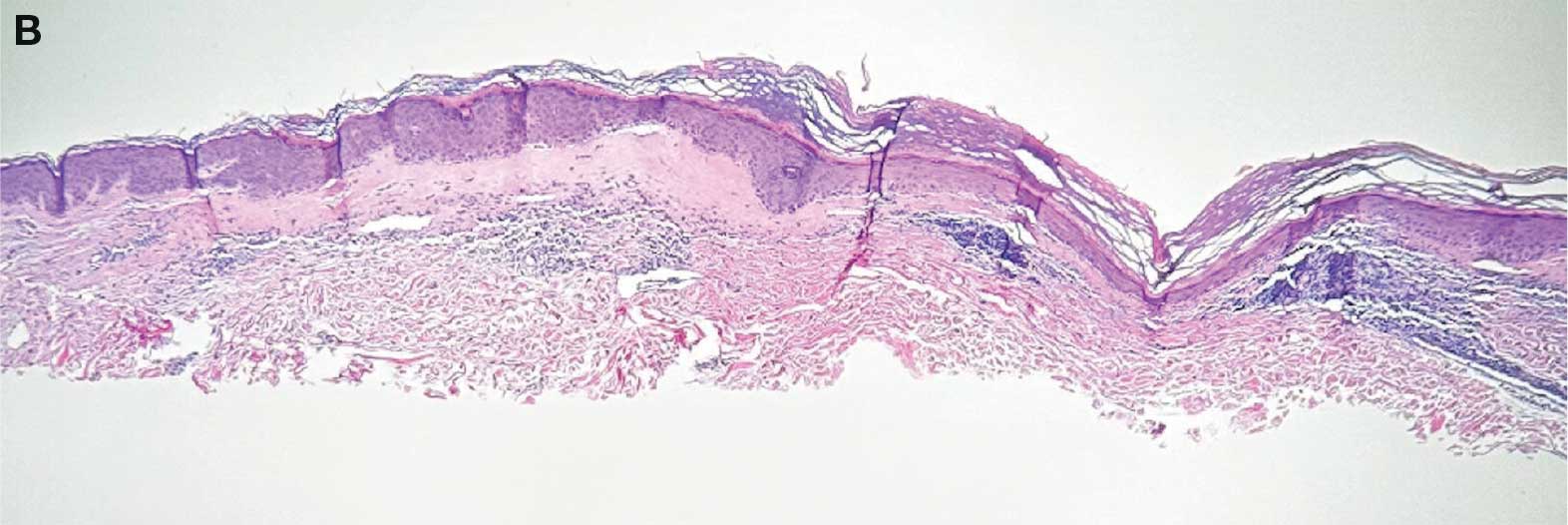
showing a compact corneal layer with a pale papillary
dermis and an underlying lymphocytic infiltrate. These
findings give the “red, white, and blue” appearance.
Low power 20× magnification.
nsbp;
Does Ethnicity Affect Skin Cancer Risk?
Does Ethnicity Affect Skin Cancer Risk?
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Does Ethnicity Affect Skin Cancer Risk?
Does Ethnicity Affect Skin Cancer Risk?
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
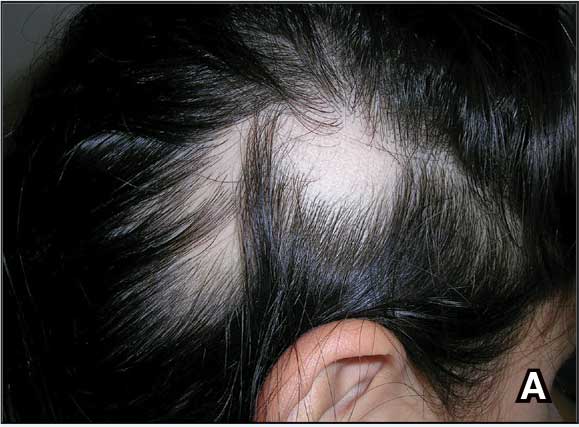
young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Data Trends 2025: Dermatology
Click here to view more from Federal Health Care Data Trends 2025.
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.
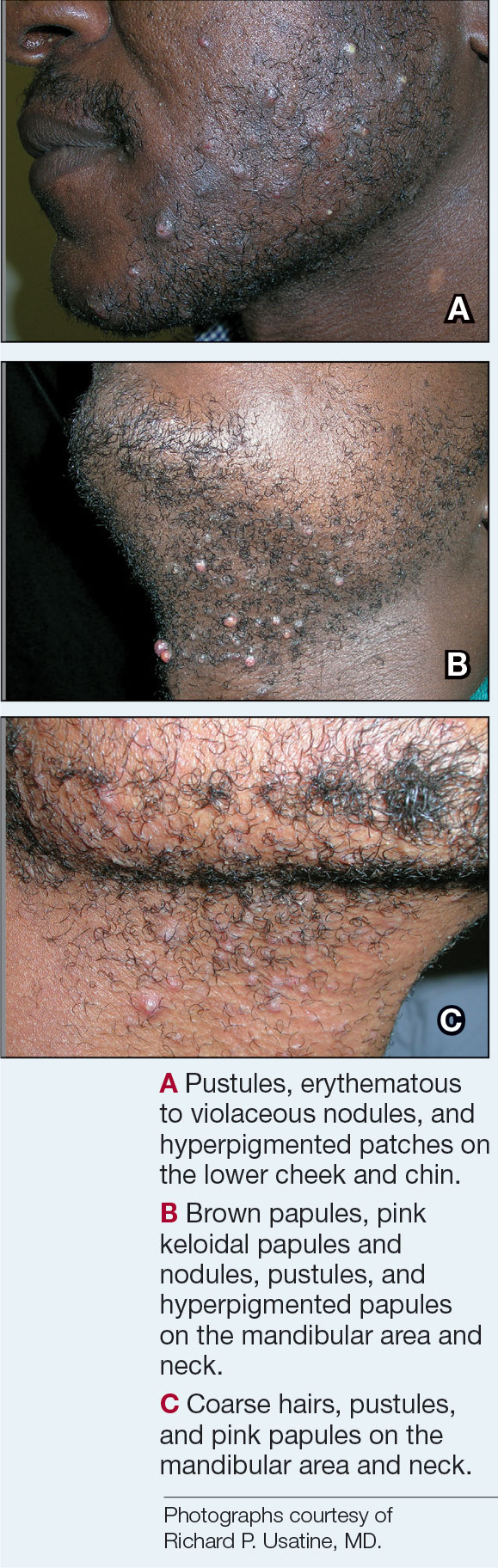
Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 PFB is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
PFB is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 PFB is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
PFB is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 PFB is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
PFB is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
End of Medical Exemptions for Grooming Impacts Black Soldiers
End of Medical Exemptions for Grooming Impacts Black Soldiers
The US military has revised its grooming standards to remove medical exemptions for male facial hair, a policy change that may put careers at risk for thousands of service members. According to the updated guidelines, all soldiers must be clean-shaven on duty when in uniform or civilian clothes, with temporary exemptions for medical reasons and permanent exemptions for religious accommodations.
The Army is the latest service branch to update its guidelines about beards: Soldiers with skin conditions will no longer be granted permanent medical waivers that allow them to avoid shaving. The Air Force and Space Force updated their guidance on grooming waivers in January, as did the Marine Corps in March.
Defense Secretary Pete Hegseth, who ordered the guideline review, focused on grooming and appearance. In a Feb. 7 townhall with troops and department employees, he said, “It starts with the basic stuff, right? It’s grooming standards and uniform standards and training standards, fitness standards, all of that matters.”
Hegseth compared not enforcing grooming standards to the “broken windows” theory of policing: “I’m not saying if you violate grooming standards, you’re a criminal. The analogy is incomplete. But if you violate the small stuff and you allow it to happen, it creates a culture where the big stuff, you’re not held accountable for.”
The policy changes are particularly significant for soldiers who grow beards because they suffer from pseudofolliculitis barbae (PFB), an often-painful genetic condition that causes ingrown hairs. PFB produces flesh-colored or red follicular papules, which can be itchy, tender, and may bleed when shaved. Even if they heal, the lesions may lead to postinflammatory hyperpigmentation, scarring (including keloid scarring), and abscess.
Although the updated standards affect all service members with beards, they draw ire from those who claim the rules disproportionately affect men of African descent. Up to 60% of Black men have PFB, according to the American Osteopathic College of Dermatology. According to the US Department of Defense (DoD) 2023 Demographics: Profile of the Military Community, service members who self-identify as Black or African American make up 17% of the total DoD military force (N = 2,034,426). Of 1,273,382 active-duty members, 18% are Black. Of 1,038,909 active-duty enlisted members, 20% are Black, and 9% of 234,473 active-duty officers are Black.
“Almost 65% of the US Air Force shaving waivers are held by Black men. And PFB is one of the most common reasons,” DanTasia Welch, MS, told Federal Practitioner. She, along with Richard P. Usatine, MD, and Candrice R. Heath, MD, wrote a recent review of the impact of PFB that was published in Federal Practitioner.
“It is almost exclusively found in men of African descent,” Usatine said. “That just means if you have a policy that affects people with this condition, you are basically aiming that policy directly at Black men.”
“Pseudofolliculitis barbae, a lot of that just has to do with your shaving technique is what we’ve determined,” Steve Warren, an Army spokesman, told reporters in early July. “A vast majority of minority soldiers, African American soldiers, are within the standards all the time.”
Usatine disagreed: “[PFB] is genetic, and whether you shave with or against the direction of the hairs, the problem is still there, and you can't just shave it away by ‘shaving correctly.’ They're going after one racial/ethnic group who has this problem, because almost everyone that has the problem is of African descent.”
The most effective management for PFB is to discontinue shaving. Grooming techniques and topical medications can be effective in treating mild-to-moderate cases of PFB, but more severe cases respond best to laser therapy. The Army, Navy, and Marine Corps advise laser therapy as a treatment option, but it has drawbacks. It is expensive and coded as a cosmetic procedure, and patients also may not have access to specialists experienced in performing the procedure in people with darker skin tones. Some patients may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.
A survey of Air Force members with 10,383 responses suggested that the men who had medical shaving waivers experienced longer times to promotion than those with no waiver. Most in the waiver group were Black or African American.
The branches have handled the rule change in different ways. The Air Force, for example, which began tightening its standards on uniform and shaving waivers in January 2025, grants long-term shaving waivers only to airmen or guardians who have severe cases of PFB following consultation with medical practitioners. Air Force Surgeon General Lt. Gen. John DeGoes said in a video that the department’s 2020 (now expired) policy allowing 5-year shaving waivers did not give clinicians enough clarity on diagnosis by not differentiating between PFB and shaving irritation.
“They are 2 different things,” DeGoes said. “Ensuring a standardized approach to managing PFB is essential. And it is crucial that we provide consistent and effective care to our service members, enabling them to meet grooming standards while managing their condition.”
The new grooming policies leave many service members in an uncomfortable quandary: Keep the beard, run the risk of getting kicked out; keep shaving and put your skin and health at risk for complications; or receive laser treatment and have to deal with lack of beard hair after leaving the military.
Simply changing the rules isn’t enough. Candrice Heath, MD, told Federal Practitioner, “You need to always strike a balance. One of those points that’s always raised is about the facial equipment that's needed to protect during times of war.”
Heath called for more research funding to develop equipment, so people can have some facial hair if needed. “There is an opportunity to not just say, hey, this is an issue, but there's an opportunity for innovation here, to really think about it this problem in a different way, so that we are solution-focused.”
The US military has revised its grooming standards to remove medical exemptions for male facial hair, a policy change that may put careers at risk for thousands of service members. According to the updated guidelines, all soldiers must be clean-shaven on duty when in uniform or civilian clothes, with temporary exemptions for medical reasons and permanent exemptions for religious accommodations.
The Army is the latest service branch to update its guidelines about beards: Soldiers with skin conditions will no longer be granted permanent medical waivers that allow them to avoid shaving. The Air Force and Space Force updated their guidance on grooming waivers in January, as did the Marine Corps in March.
Defense Secretary Pete Hegseth, who ordered the guideline review, focused on grooming and appearance. In a Feb. 7 townhall with troops and department employees, he said, “It starts with the basic stuff, right? It’s grooming standards and uniform standards and training standards, fitness standards, all of that matters.”
Hegseth compared not enforcing grooming standards to the “broken windows” theory of policing: “I’m not saying if you violate grooming standards, you’re a criminal. The analogy is incomplete. But if you violate the small stuff and you allow it to happen, it creates a culture where the big stuff, you’re not held accountable for.”
The policy changes are particularly significant for soldiers who grow beards because they suffer from pseudofolliculitis barbae (PFB), an often-painful genetic condition that causes ingrown hairs. PFB produces flesh-colored or red follicular papules, which can be itchy, tender, and may bleed when shaved. Even if they heal, the lesions may lead to postinflammatory hyperpigmentation, scarring (including keloid scarring), and abscess.
Although the updated standards affect all service members with beards, they draw ire from those who claim the rules disproportionately affect men of African descent. Up to 60% of Black men have PFB, according to the American Osteopathic College of Dermatology. According to the US Department of Defense (DoD) 2023 Demographics: Profile of the Military Community, service members who self-identify as Black or African American make up 17% of the total DoD military force (N = 2,034,426). Of 1,273,382 active-duty members, 18% are Black. Of 1,038,909 active-duty enlisted members, 20% are Black, and 9% of 234,473 active-duty officers are Black.
“Almost 65% of the US Air Force shaving waivers are held by Black men. And PFB is one of the most common reasons,” DanTasia Welch, MS, told Federal Practitioner. She, along with Richard P. Usatine, MD, and Candrice R. Heath, MD, wrote a recent review of the impact of PFB that was published in Federal Practitioner.
“It is almost exclusively found in men of African descent,” Usatine said. “That just means if you have a policy that affects people with this condition, you are basically aiming that policy directly at Black men.”
“Pseudofolliculitis barbae, a lot of that just has to do with your shaving technique is what we’ve determined,” Steve Warren, an Army spokesman, told reporters in early July. “A vast majority of minority soldiers, African American soldiers, are within the standards all the time.”
Usatine disagreed: “[PFB] is genetic, and whether you shave with or against the direction of the hairs, the problem is still there, and you can't just shave it away by ‘shaving correctly.’ They're going after one racial/ethnic group who has this problem, because almost everyone that has the problem is of African descent.”
The most effective management for PFB is to discontinue shaving. Grooming techniques and topical medications can be effective in treating mild-to-moderate cases of PFB, but more severe cases respond best to laser therapy. The Army, Navy, and Marine Corps advise laser therapy as a treatment option, but it has drawbacks. It is expensive and coded as a cosmetic procedure, and patients also may not have access to specialists experienced in performing the procedure in people with darker skin tones. Some patients may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.
A survey of Air Force members with 10,383 responses suggested that the men who had medical shaving waivers experienced longer times to promotion than those with no waiver. Most in the waiver group were Black or African American.
The branches have handled the rule change in different ways. The Air Force, for example, which began tightening its standards on uniform and shaving waivers in January 2025, grants long-term shaving waivers only to airmen or guardians who have severe cases of PFB following consultation with medical practitioners. Air Force Surgeon General Lt. Gen. John DeGoes said in a video that the department’s 2020 (now expired) policy allowing 5-year shaving waivers did not give clinicians enough clarity on diagnosis by not differentiating between PFB and shaving irritation.
“They are 2 different things,” DeGoes said. “Ensuring a standardized approach to managing PFB is essential. And it is crucial that we provide consistent and effective care to our service members, enabling them to meet grooming standards while managing their condition.”
The new grooming policies leave many service members in an uncomfortable quandary: Keep the beard, run the risk of getting kicked out; keep shaving and put your skin and health at risk for complications; or receive laser treatment and have to deal with lack of beard hair after leaving the military.
Simply changing the rules isn’t enough. Candrice Heath, MD, told Federal Practitioner, “You need to always strike a balance. One of those points that’s always raised is about the facial equipment that's needed to protect during times of war.”
Heath called for more research funding to develop equipment, so people can have some facial hair if needed. “There is an opportunity to not just say, hey, this is an issue, but there's an opportunity for innovation here, to really think about it this problem in a different way, so that we are solution-focused.”
The US military has revised its grooming standards to remove medical exemptions for male facial hair, a policy change that may put careers at risk for thousands of service members. According to the updated guidelines, all soldiers must be clean-shaven on duty when in uniform or civilian clothes, with temporary exemptions for medical reasons and permanent exemptions for religious accommodations.
The Army is the latest service branch to update its guidelines about beards: Soldiers with skin conditions will no longer be granted permanent medical waivers that allow them to avoid shaving. The Air Force and Space Force updated their guidance on grooming waivers in January, as did the Marine Corps in March.
Defense Secretary Pete Hegseth, who ordered the guideline review, focused on grooming and appearance. In a Feb. 7 townhall with troops and department employees, he said, “It starts with the basic stuff, right? It’s grooming standards and uniform standards and training standards, fitness standards, all of that matters.”
Hegseth compared not enforcing grooming standards to the “broken windows” theory of policing: “I’m not saying if you violate grooming standards, you’re a criminal. The analogy is incomplete. But if you violate the small stuff and you allow it to happen, it creates a culture where the big stuff, you’re not held accountable for.”
The policy changes are particularly significant for soldiers who grow beards because they suffer from pseudofolliculitis barbae (PFB), an often-painful genetic condition that causes ingrown hairs. PFB produces flesh-colored or red follicular papules, which can be itchy, tender, and may bleed when shaved. Even if they heal, the lesions may lead to postinflammatory hyperpigmentation, scarring (including keloid scarring), and abscess.
Although the updated standards affect all service members with beards, they draw ire from those who claim the rules disproportionately affect men of African descent. Up to 60% of Black men have PFB, according to the American Osteopathic College of Dermatology. According to the US Department of Defense (DoD) 2023 Demographics: Profile of the Military Community, service members who self-identify as Black or African American make up 17% of the total DoD military force (N = 2,034,426). Of 1,273,382 active-duty members, 18% are Black. Of 1,038,909 active-duty enlisted members, 20% are Black, and 9% of 234,473 active-duty officers are Black.
“Almost 65% of the US Air Force shaving waivers are held by Black men. And PFB is one of the most common reasons,” DanTasia Welch, MS, told Federal Practitioner. She, along with Richard P. Usatine, MD, and Candrice R. Heath, MD, wrote a recent review of the impact of PFB that was published in Federal Practitioner.
“It is almost exclusively found in men of African descent,” Usatine said. “That just means if you have a policy that affects people with this condition, you are basically aiming that policy directly at Black men.”
“Pseudofolliculitis barbae, a lot of that just has to do with your shaving technique is what we’ve determined,” Steve Warren, an Army spokesman, told reporters in early July. “A vast majority of minority soldiers, African American soldiers, are within the standards all the time.”
Usatine disagreed: “[PFB] is genetic, and whether you shave with or against the direction of the hairs, the problem is still there, and you can't just shave it away by ‘shaving correctly.’ They're going after one racial/ethnic group who has this problem, because almost everyone that has the problem is of African descent.”
The most effective management for PFB is to discontinue shaving. Grooming techniques and topical medications can be effective in treating mild-to-moderate cases of PFB, but more severe cases respond best to laser therapy. The Army, Navy, and Marine Corps advise laser therapy as a treatment option, but it has drawbacks. It is expensive and coded as a cosmetic procedure, and patients also may not have access to specialists experienced in performing the procedure in people with darker skin tones. Some patients may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.
A survey of Air Force members with 10,383 responses suggested that the men who had medical shaving waivers experienced longer times to promotion than those with no waiver. Most in the waiver group were Black or African American.
The branches have handled the rule change in different ways. The Air Force, for example, which began tightening its standards on uniform and shaving waivers in January 2025, grants long-term shaving waivers only to airmen or guardians who have severe cases of PFB following consultation with medical practitioners. Air Force Surgeon General Lt. Gen. John DeGoes said in a video that the department’s 2020 (now expired) policy allowing 5-year shaving waivers did not give clinicians enough clarity on diagnosis by not differentiating between PFB and shaving irritation.
“They are 2 different things,” DeGoes said. “Ensuring a standardized approach to managing PFB is essential. And it is crucial that we provide consistent and effective care to our service members, enabling them to meet grooming standards while managing their condition.”
The new grooming policies leave many service members in an uncomfortable quandary: Keep the beard, run the risk of getting kicked out; keep shaving and put your skin and health at risk for complications; or receive laser treatment and have to deal with lack of beard hair after leaving the military.
Simply changing the rules isn’t enough. Candrice Heath, MD, told Federal Practitioner, “You need to always strike a balance. One of those points that’s always raised is about the facial equipment that's needed to protect during times of war.”
Heath called for more research funding to develop equipment, so people can have some facial hair if needed. “There is an opportunity to not just say, hey, this is an issue, but there's an opportunity for innovation here, to really think about it this problem in a different way, so that we are solution-focused.”
End of Medical Exemptions for Grooming Impacts Black Soldiers
End of Medical Exemptions for Grooming Impacts Black Soldiers
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.
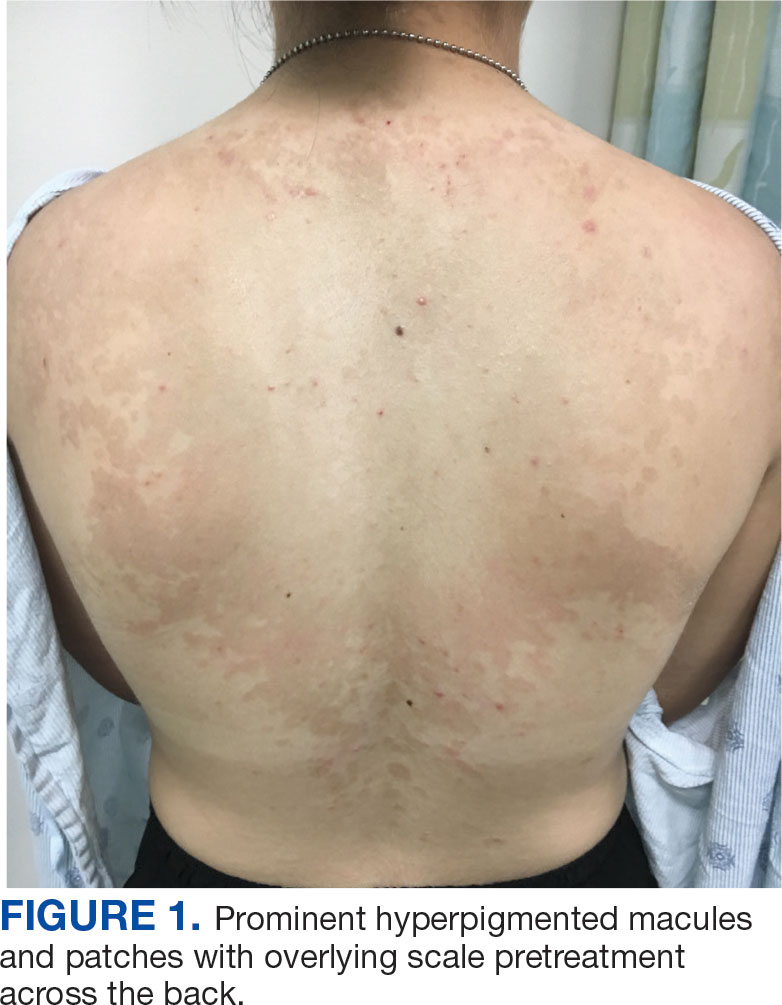
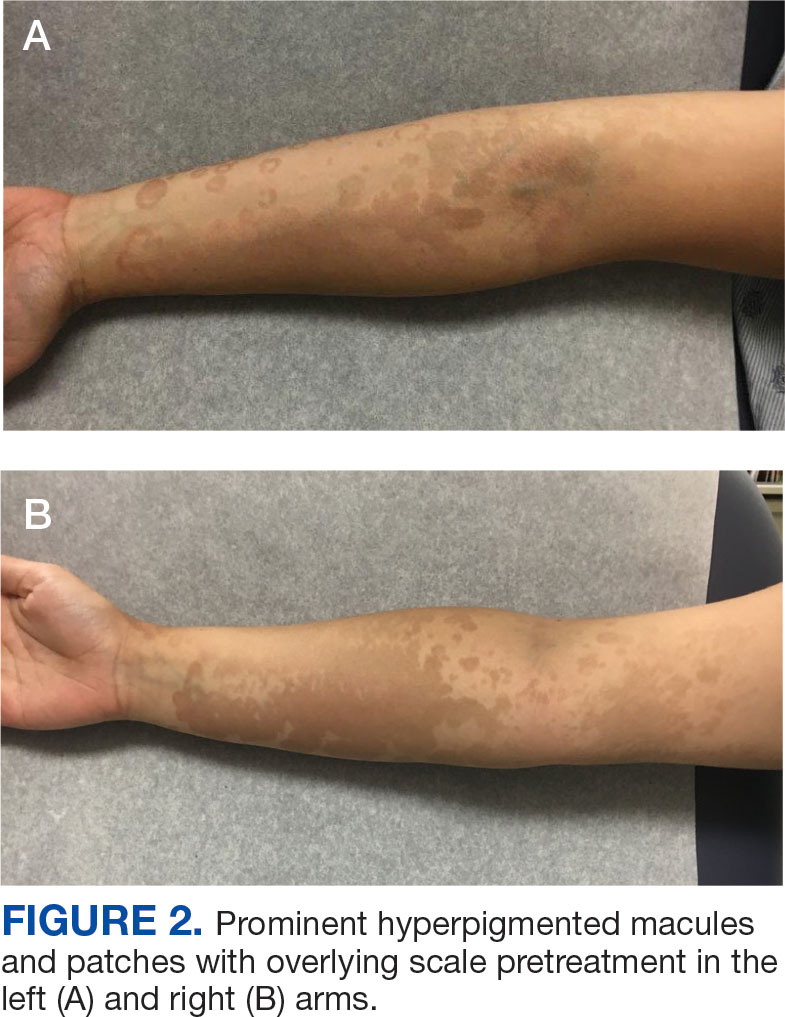
The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.
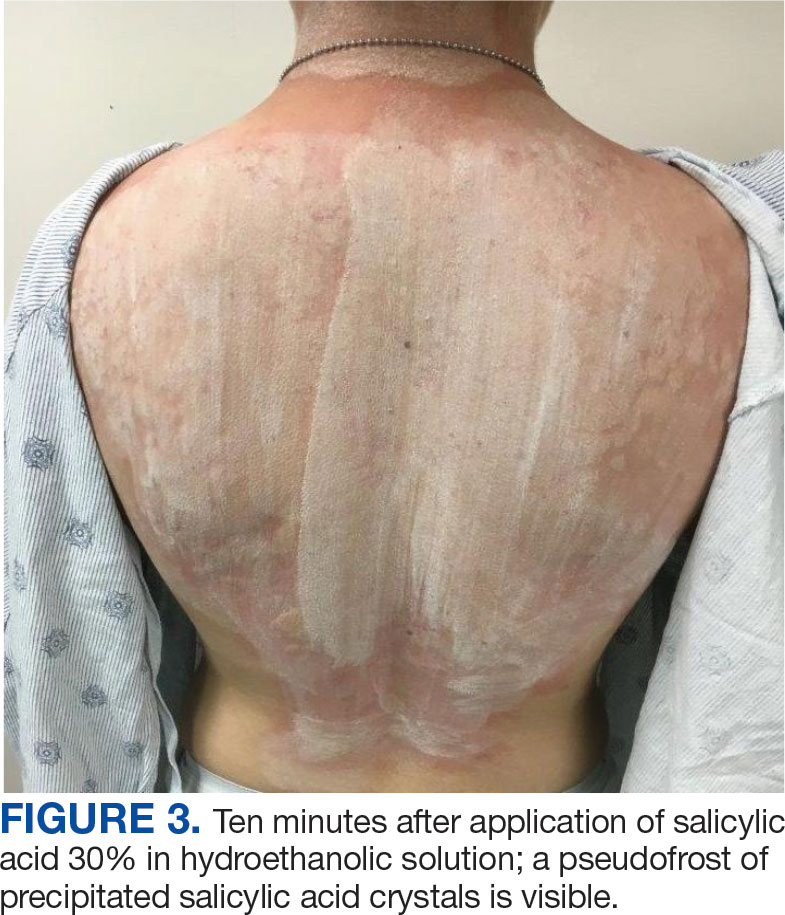
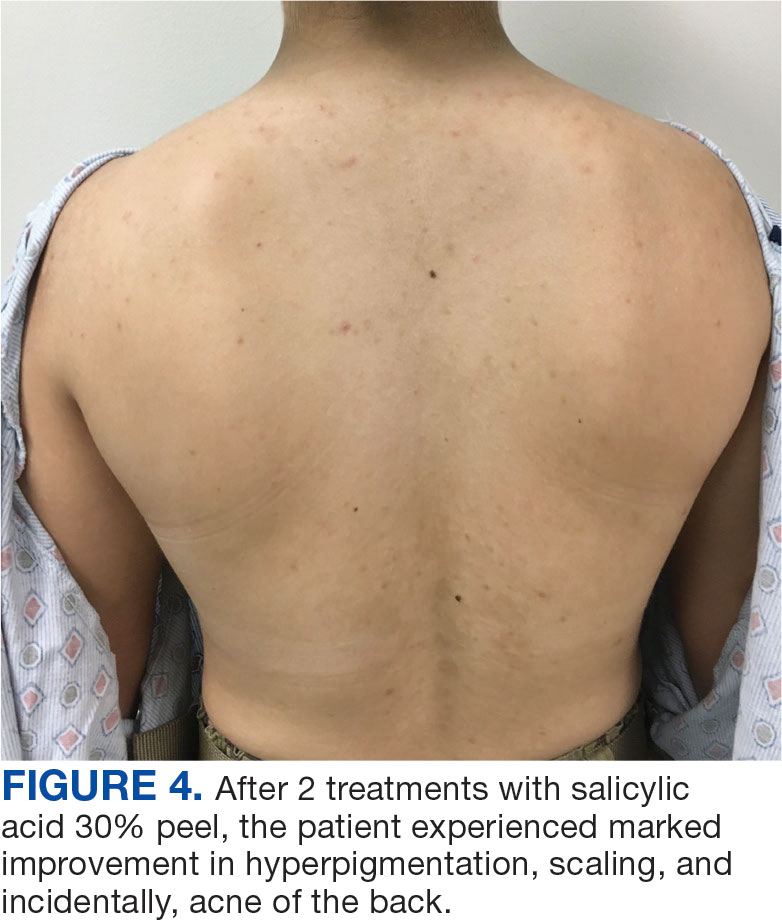
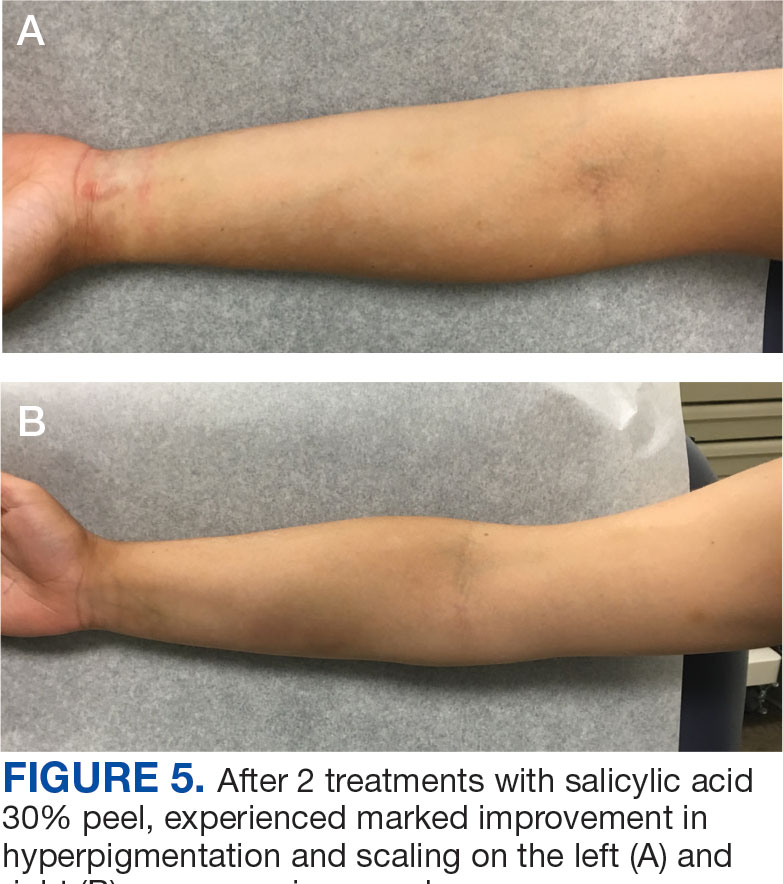
Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
A 26-year-old male with Fitzpatrick skin type II presented for evaluation in the dermatology clinic after being referred by his primary care practitioner (PCP) with a complaint of spider veins. The patient reported a lifelong history of blue subcutaneous nodules that initially appeared on his face during childhood but have since involved his trunk and upper and lower extremities. The patient reported that some of the nodules were painful and increased in size with exercise. His medical history was unremarkable with no other chronic conditions or daily medication use. The patient reported no gastrointestinal (GI) symptoms, melena, or hematochezia. The patient’s mother had similar nodules but his 7 siblings did not.
Upon physical examination, numerous blue subcutaneous nodules, 2 to 8 mm in size, were scattered across his trunk, and proximal and distal extremities were present (Figure 1). The physical examination was otherwise unremarkable. Upon discussing differential diagnosis of these lesions with the patient, he was amenable to a punch biopsy for further diagnostic clarity (Figure 2).