User login
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance 30 mL/min | Creatinine clearance 30 mL/min | Creatinine clearance 25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, 5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| 30 mL/min | 1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | 0.001 | 2.1 | 2.4 | 0.88 | 0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | 0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | 0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | 0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | 0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | 0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | 0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | 0.001 | 3.15b | 2.16b | 0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR 57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
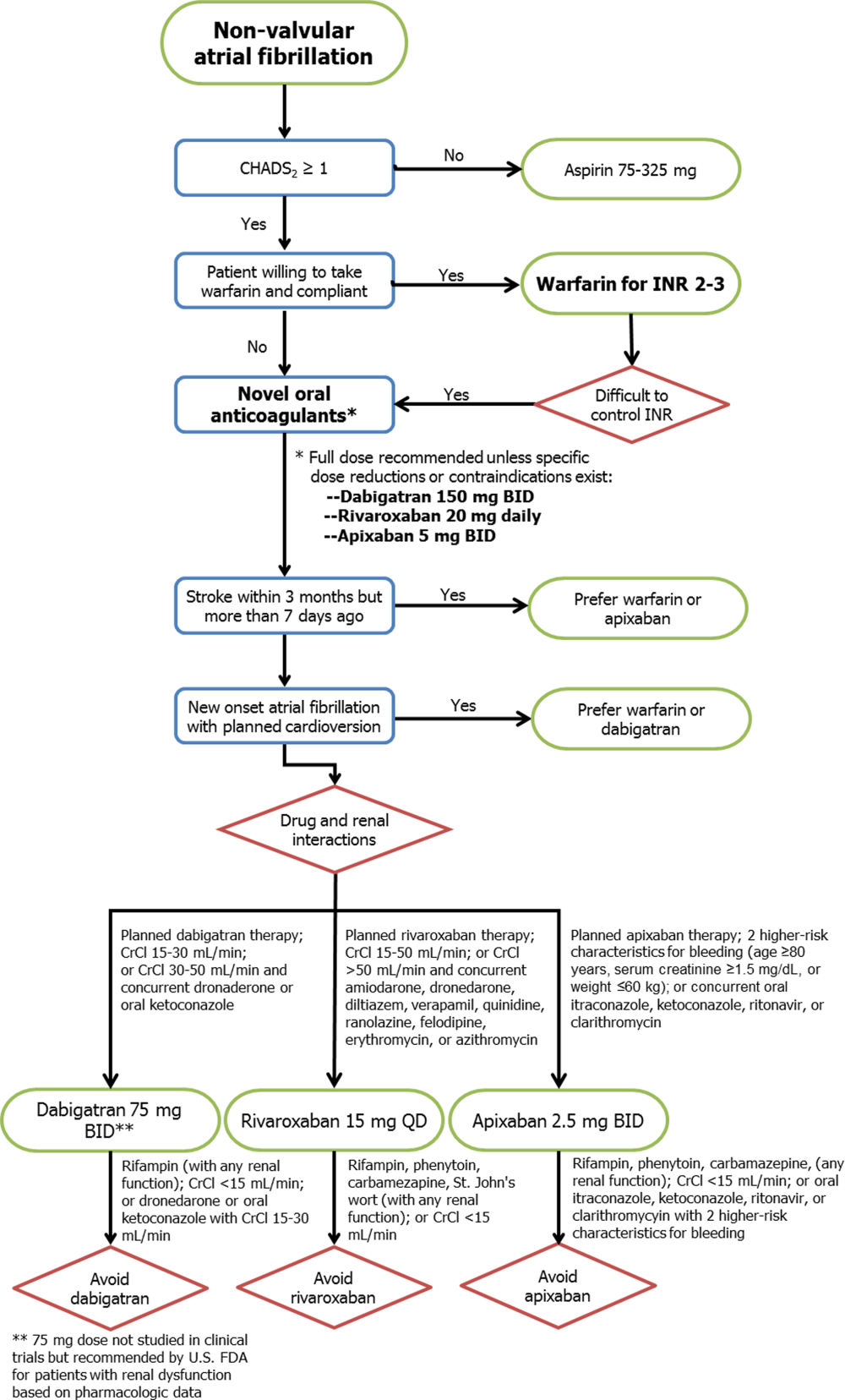
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]
Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance 30 mL/min | Creatinine clearance 30 mL/min | Creatinine clearance 25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, 5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| 30 mL/min | 1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | 0.001 | 2.1 | 2.4 | 0.88 | 0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | 0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | 0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | 0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | 0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | 0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | 0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | 0.001 | 3.15b | 2.16b | 0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR 57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]

Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance 30 mL/min | Creatinine clearance 30 mL/min | Creatinine clearance 25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, 5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| 30 mL/min | 1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | 0.001 | 2.1 | 2.4 | 0.88 | 0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | 0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | 0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | 0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | 0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | 0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | 0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | 0.001 | 3.15b | 2.16b | 0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR 57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]

Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
