User login
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
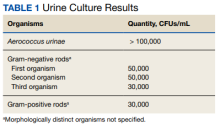
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
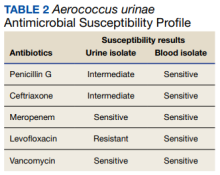
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312